PIONEERING THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health

In-Depth Understanding of Disease
June 18, 2021
It’s hard to fix something without knowing exactly what’s gone wrong. That’s why scientists at the University of Utah Health dig deep into the origins of disease, working to discern exactly what developmental pathways, cellular communications, or molecular interactions have gone awry to give rise to patients’ symptoms. By uncovering the biological mechanisms behind disease, they are paving the way to clearer diagnoses, better treatments, and even prevention.
More than 575,000 people each year develop cerebral malaria, a severe form of malaria that causes coma. Even with treatment, it kills 20 percent of those who are infected, and its effects on the brain can cause long-term problems for survivors, such as seizures and mental health disorders. Young children in sub-Saharan Africa are disproportionally affected by this disease.
The severity of cerebral malaria seems to be, at least in part, due to inflammation that the immune system deploys to fight the malaria parasite. Pathology professor Tracey Lamb, Ph.D., and colleagues have discovered that signaling molecules called Eph receptors contribute to this inflammation, making this condition worse. Working with researchers at the Pasteur Institute in Cameroon, Lamb’s team has found that the Eph receptor EphA2 is key to the breakdown of the blood-brain barrier that occurs during cerebral malaria. A related molecule, EphB2, plays a key role in liver fibrosis, an accumulation of scar tissue in the liver that is another consequence of a strong inflammatory response to the malaria parasite. They have found that they can protect the brain and liver in infected mice by blocking or eliminating these Eph receptors, suggesting a strategy for preventing some of the most severe symptoms found in malaria.
Learn more about this discovery.
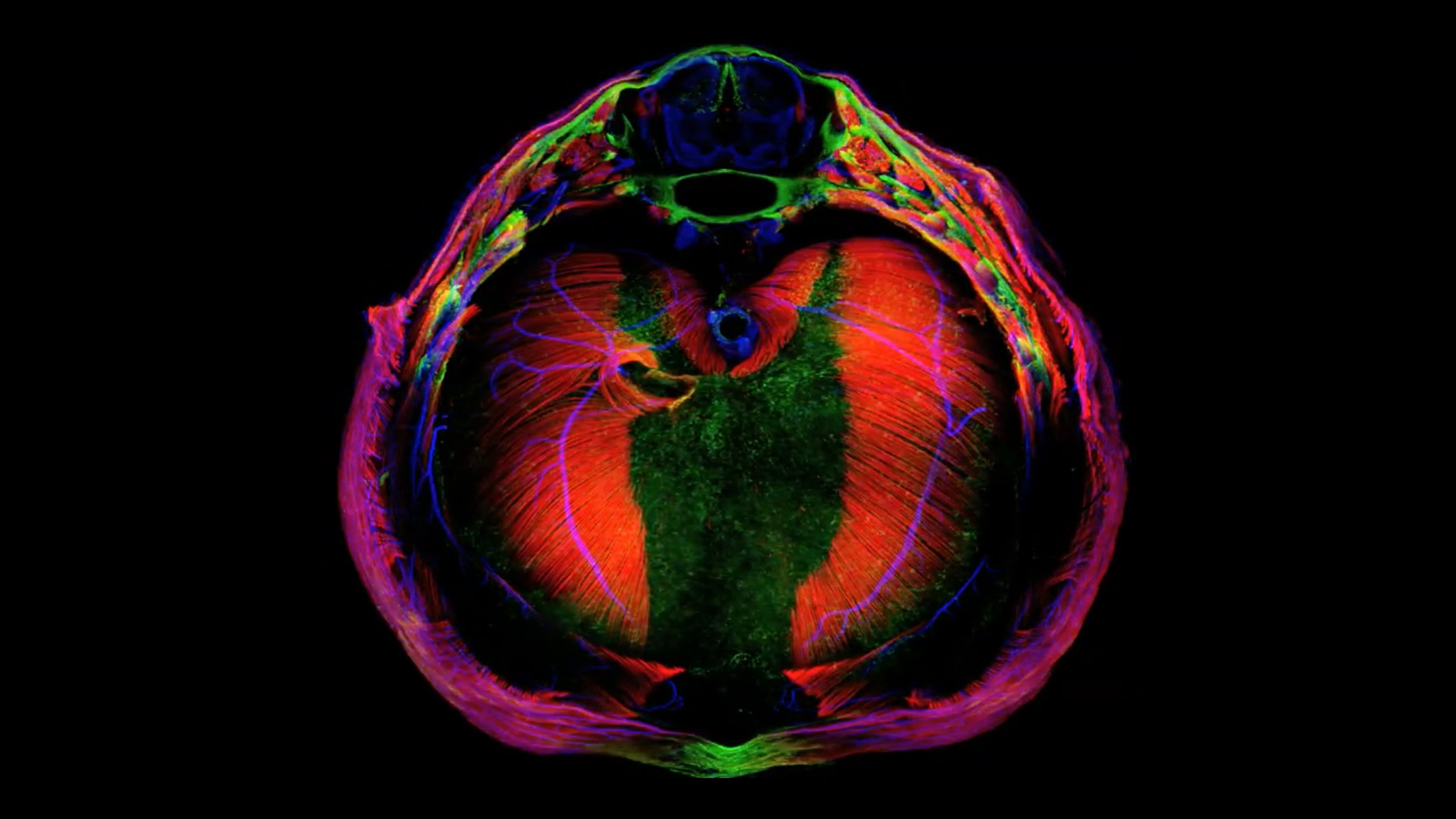
Structural abnormalities in the heart can cause serious illness in infants and children, or they can go undetected until they cause problems later in life. There are many ways things can go wrong as the heart’s valves and chambers take shape in a growing embryo, but H. Joseph Yost, Ph.D., and colleagues have traced two seemingly unrelated heart problems—heart failure in adults and the congenital heart defects associated with Kabuki syndrome, a rare disorder whose symptoms begin at birth—to the same developmental pathway.
By studying how the heart develops in zebrafish, Yost’s team in the Department of Neurobiology discovered that embryonic heart-cell precursors use a signaling pathway called Notch to guide this process. If certain cells are missing or unable to activate the Notch pathway early in development, fish experience heart failure when subjected to cardiac stress as adults. Like people with a condition called hypertrophic cardiomyopathy, these fish have hearts whose walls are thicker than those of a healthy heart. Problems with Notch also appear to be to blame for heart defects that are common among people with Kabuki Syndrome. In zebrafish with a genetic mutation that causes the disorder, the Notch pathway is overactive—but Yost’s team was able to restore normal heart development by inhibiting Notch signaling. Understanding the developmental origins of these heart conditions will help researchers and clinicians find ways to better diagnose and manage them.
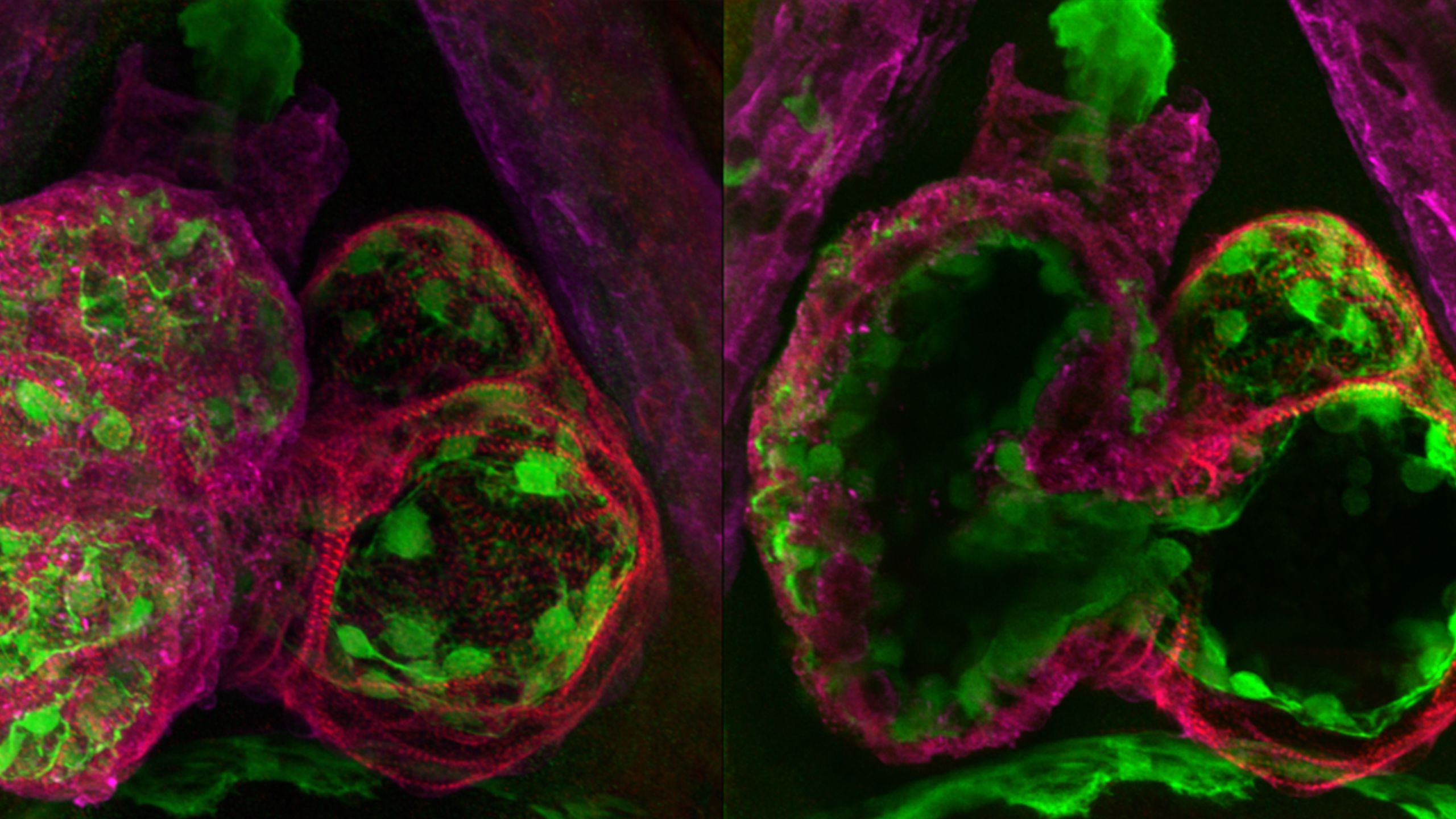
The diaphragm is essential: It contracts and expands to help the lungs breathe and it separates the heart and lungs from the abdominal cavity, ensuring they have the space they need. A common birth defect known as a congenital diaphragmatic hernia (CDH) compromises this barrier, allowing digestive organs to protrude into the chest cavity and interfere with the lungs. CDH is life-threatening. Even with surgery, about half of babies born with the condition do not survive.
To find out what goes wrong to cause CDH, a team led by geneticist Gabrielle Kardon, Ph.D., studied how the diaphragm normally develops. By following the process in embryonic mice, they found that developing connective tissue, which will eventually anchor the muscle of the diaphragm to bones and tendons, is important for directing the muscle’s development. A genetic mutation associated with CDH impairs the connective tissue’s communication with the developing muscle, leading to a region where the diaphragm is made only of connective tissue. As a growing embryo’s liver presses on this weak spot, the diaphragm gives way. By uncovering how CDH can develop, the research has opened a door to potential preventative treatments.
Polycystic kidney disease is a common, untreatable disorder that begins with mutations that affect a critical molecular channel in the body’s cells. People with these mutations develop cysts that predominantly affect the kidneys. The cysts interfere with the kidneys’ ability to filter waste from the blood, and many people with polycystic kidney disease eventually need either dialysis or a kidney transplant. The disease can also cause problems in the liver, pancreas, and cardiovascular system. Until U of U Health scientists captured the first high-resolution image of the polycystic kidney disease channel, researchers had little idea how the mutations that cause the disease impact cellular function–or how to go about correcting the problem.
Because they are usually embedded within cell membranes, channel proteins are notoriously difficult to visualize. But researchers led by biochemist Erhu Cao, Ph.D., managed to bring a component of the channel called PKD2 into sharp focus. In the lab, Cao’s team nestled PKD2 within tiny islands of fat that mimic a cell membrane. Then they captured an image of the structure that was so detailed they were able to see how the protein is organized and infer how it works. They learned that disease-causing mutations fall within regions that are important for holding the channel together, and likely cause the whole thing to fall apart. This knowledge will help guide researchers as they work to develop potential treatments for polycystic kidney disease.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist for the original collection of discoveries.
Written by: Jennifer Michalowski
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Will Dere, Chris Hill, Amy Tanner