PIONEERING THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health

Tuning Up the Cell’s Engines
July 18, 2021
To keep our bodies running smoothly, people need a continuous supply of food. The body breaks down food into component molecules, and cells convert these nutrients into energy. At U of U Health, scientists are delving into the complex network of biochemical reactions by which cells convert food into energy — the processes collectively called metabolism — to gain valuable insights into health and disease.
One way or another, cellular metabolism underpins everything that happens in the body. Changes outside the body, like a sedentary lifestyle or consuming too many calories, can shift our cells’ metabolic processes and cause disease. Metabolism can also be affected by internal changes, including genetic mutations. Mutations that turbocharge energy production can lead to unconstrained cell division and cancer. Understanding these energy processes can lead to new treatments for obesity, heart disease, diabetes, cancer and more.
Inside every cell, complex chemical reactions convert fuel molecules, such as glucose, into energy. Cancer cells need even more energy than most normal cells but also require the building blocks for their rapid duplication. Understanding how they change their metabolism to meet these needs could lead to new types of treatment.
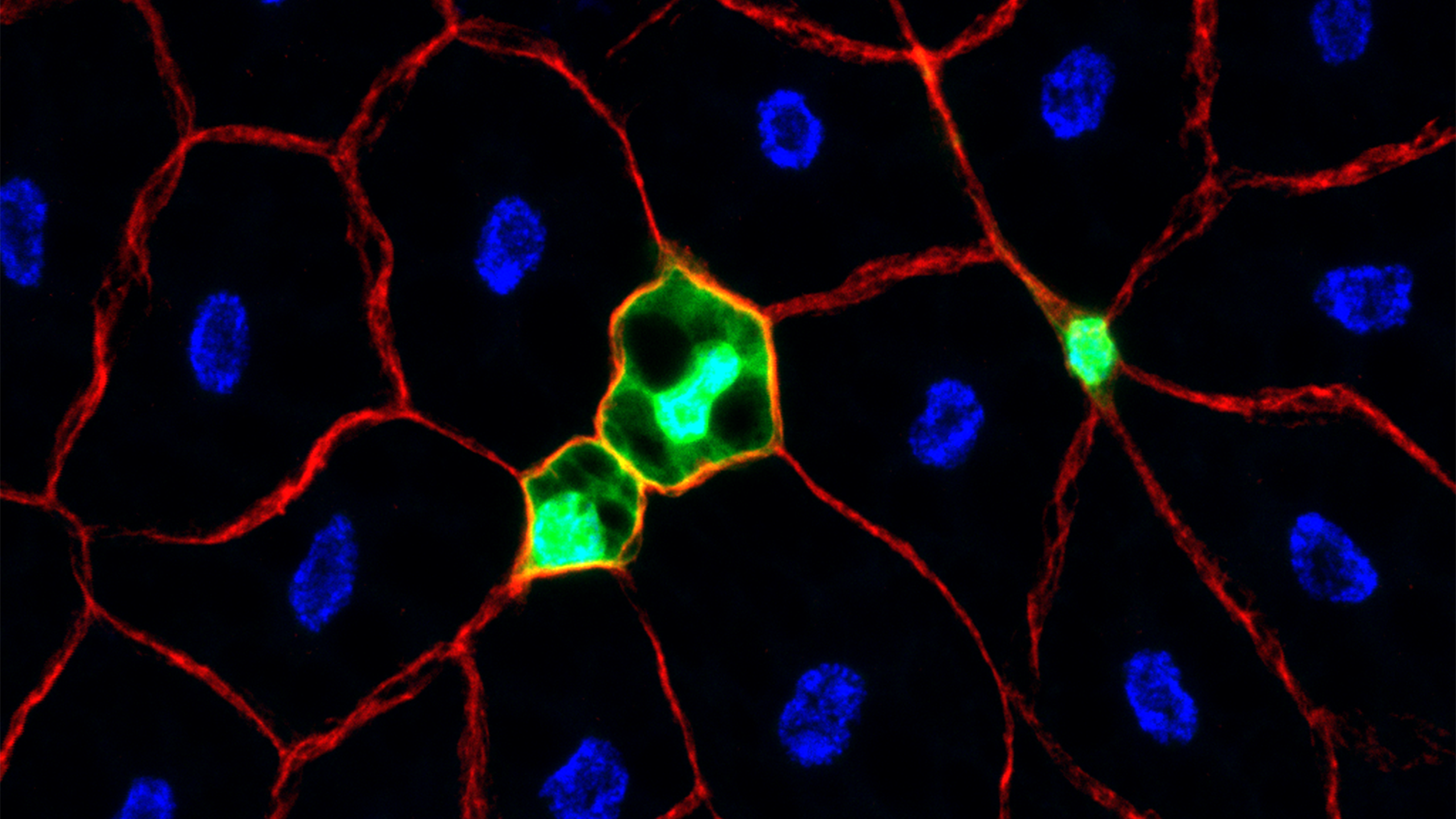
Cells obtain energy from several different types of fuel, but the simple sugar glucose is particularly important in cancer cells. Normally, cells burn sugars for energy inside a mini-organ called the mitochondria. Cancer cells prevent pyruvate from entering the mitochondria, diverting it instead to metabolic pathways to produce building blocks.
Biochemist Jared Rutter, PhD, and colleagues discovered molecules called MPCs, for “mitochondrial pyruvate carriers,” which act as doorways to let pyruvate enter the mitochondria. Their work revealed that the choice of whether to import pyruvate has far-reaching medical implications. As cancer cells accumulate genetic mutations that enable uncontrolled growth and reproduction, mutations that reduce MPC production make the tumor more aggressive and deadlier. Importantly, adding MPC back to the tumor can slow down its growth. “We hope that this information can be used to design therapies that are specifically toxic to cancer cells,” Rutter says.
Learn more about this discovery.
Molecular biologist Katsu Funai, PhD, recalls his mother injuring her ankle when she was just 44 years old. “She was really never the same after that,” Funai says. Even after several surgeries, her mobility remained limited, and she suffered from lifelong illnesses across multiple organ systems. That experience fuels Funai’s drive to understand how exercise affects cellular metabolism. “My passion for medical research is driven by my conviction that the root of all these diseases is the inactivity that she suffered since her injury.”
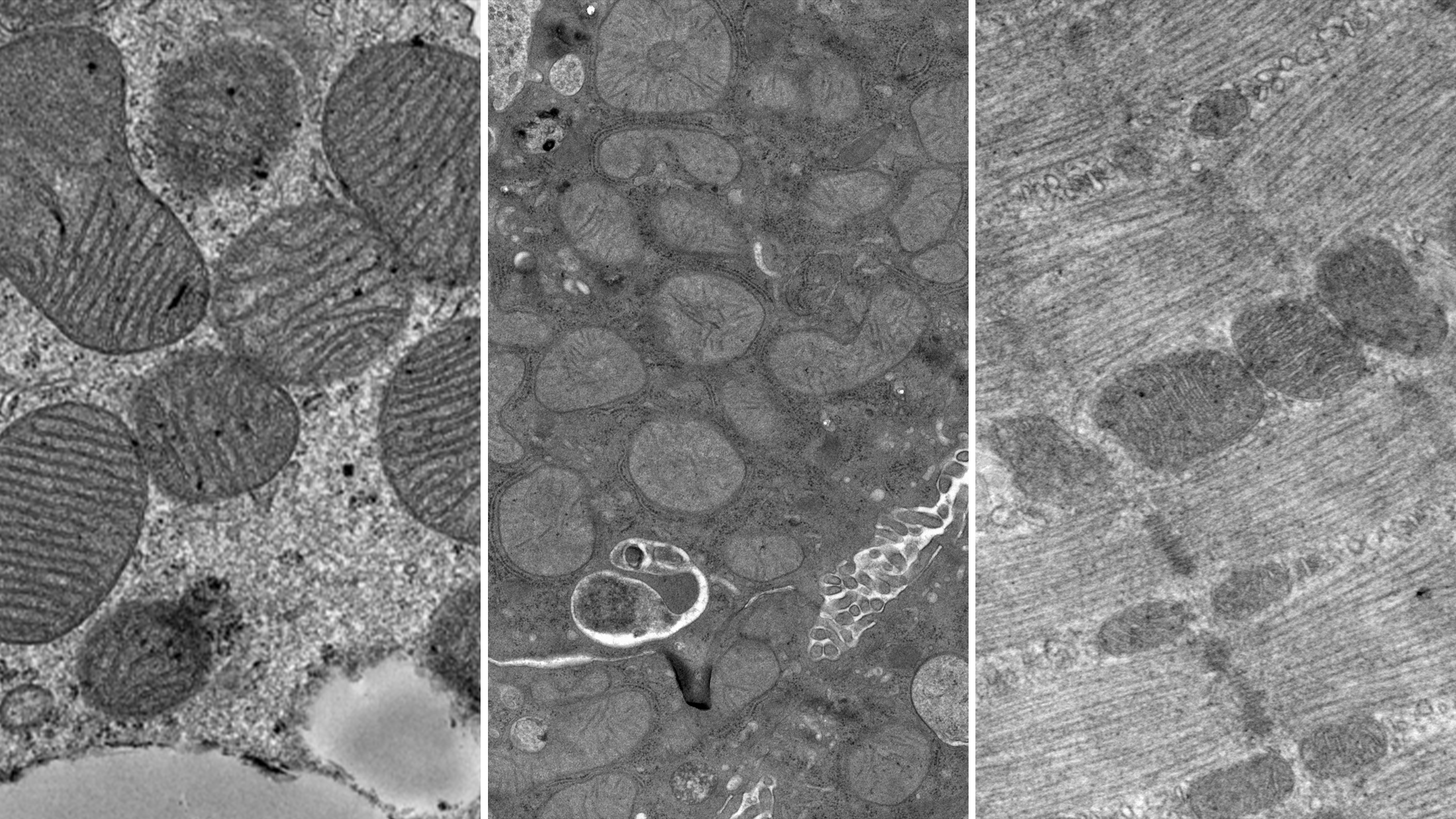
Funai and his colleagues discovered that exercise increased the amount of a certain fat molecule, called phosphatidylethanolamine (PE), in the inner membrane of the mitochondria. Having PE in the membrane made the mitochondria produce energy more efficiently. When mice were genetically engineered to make less PE than normal, their mitochondria didn’t work as well. Their muscles deteriorated, and within weeks the animals died because the muscles that control breathing stopped working.
“The changes we saw were highly robust and significant,” says Funai. His research puts lipids into a new light: addressing their role on mitochondrial function could lead to therapies for treating diabetes and heart disease.
A sedentary lifestyle can have a major impact on organ systems throughout the body. But did you know that scientists have found that fat behaves like an organ as well? More than just a static storehouse of unused calories, fat cells send and receive chemical signals, interacting with other organs and responding to changing conditions in the body. Excess fat in the abdomen, called “visceral fat,” increases risk of heart disease, stroke, diabetes, and many other medical conditions.
Sihem Boudina, PhD, studies how visceral fat forms and expands in response to excess calorie consumption. This kind of fat can increase in two ways: the fat cells can become larger, or they can summon a kind of stem cell that will spawn new fat cells. Boudina and her colleagues discovered a set of molecular signaling events that lead to fat cell growth. Learning more about how these different mechanisms work could reveal opportunities to intervene against intractable obesity or to stave off metabolic disease or cardiovascular disease.
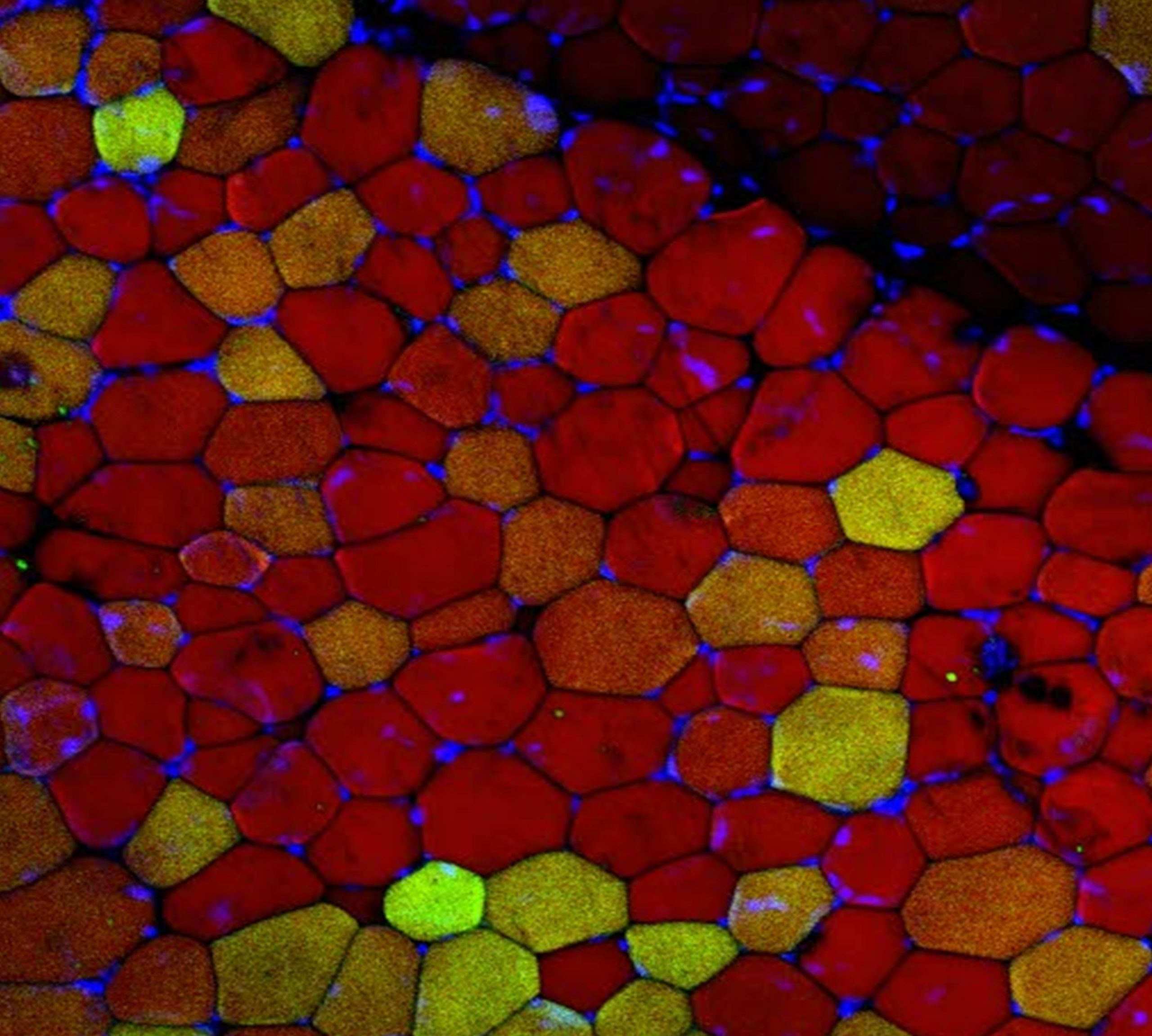
It’s good to remember that not all fat is harmful. Mammals, including mice and humans, have the ability to internally turn on the heat when the outside temperature plummets. That process, called “cold-induced thermogenesis,” burns a lot of energy, and it’s done by a certain type of fat cells.
Unlike “white fat,” which stores energy -- and makes us, well, fat -- “brown fat” burns calories to generate body heat. Biochemist Claudio Villanueva, PhD, and his colleagues discovered that in response to cold, the liver switches metabolism to provide a molecule called acylcarnitines, which brown fat burns as fuel. Feeding mice a supplement called L-carnitine enabled them to tolerate chilly conditions that would ordinarily trigger hypothermia.
Because brown fat burns calories, jump-starting the cold adaptation process may be useful not only for reducing cold sensitivity but also for fighting obesity. “If we can find a way to tell the body to expend more energy than it is taking in, the calories lost can lead to weight loss,” says Villanueva. Now, with an activator of brown fat in hand, scientists have a handle on manipulating brown fat’s power.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist and Alfred Cheung for their dedicated work compiling research discoveries.
Written by: Caroline Seydel
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Will Dere, Chris Hill, Amy Tanner