New Techniques to Reach New Frontiers
December 8, 2022
Scientific progress can be slow but steady, requiring patience, repetition, and long-term commitment. But sometimes an innovative new technique changes the way things are done. Progress accelerates and suddenly researchers find themselves exploring new frontiers. Techniques developed at U of U Health are enabling researchers to work more efficiently, study previously inaccessible aspects of biology, and glean more meaningful information from the data they already have. Their tools and methods are shared freely with the research community so everyone is better equipped to investigate and improve human health.
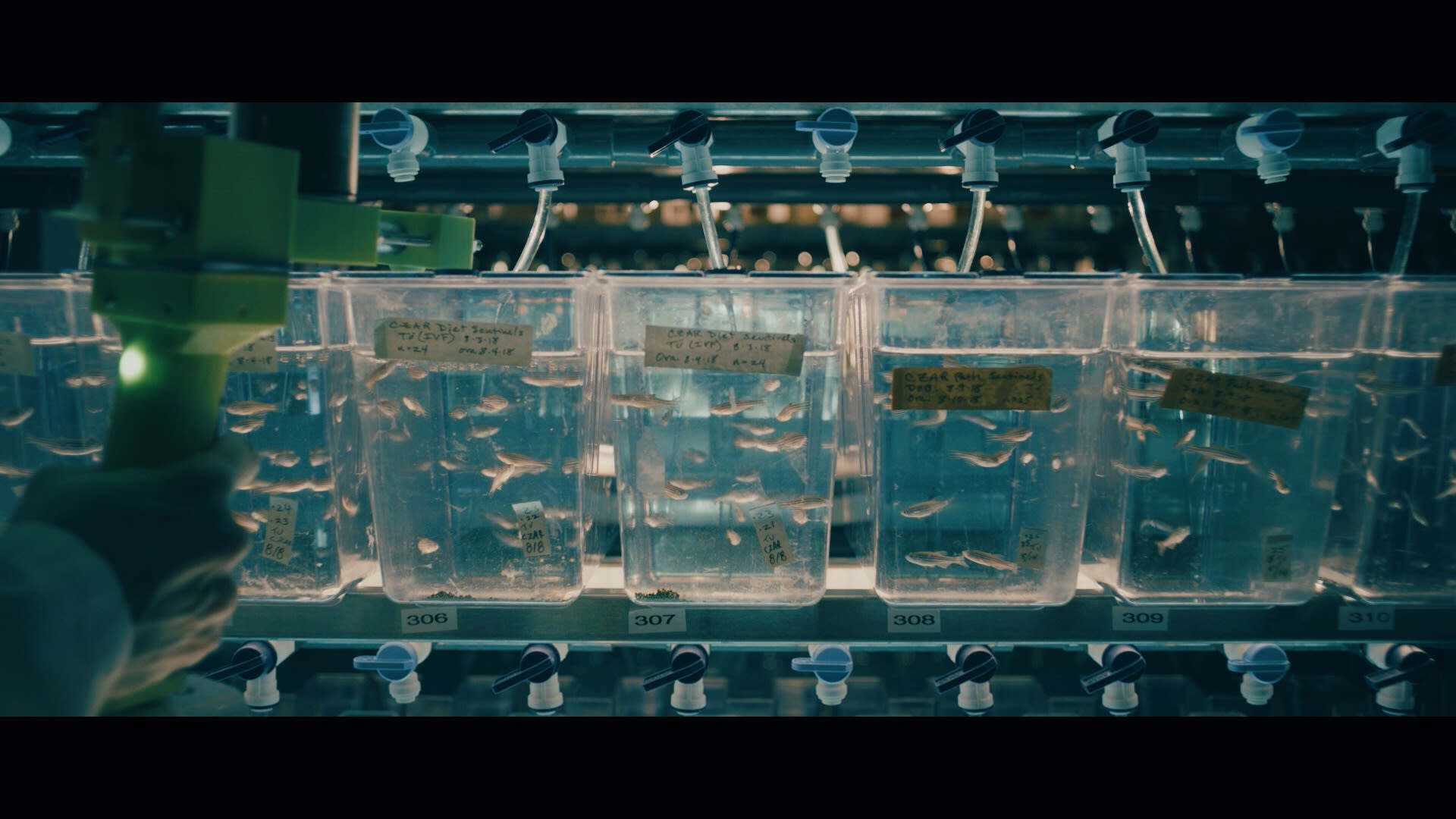
Modern biology has been revolutionized by CRISPR, a programmable system for editing DNA with speed and precision. Among its many uses is in learning about gene function. Researchers can use CRISPR to disrupt a specific gene, then study how the change impacts an organism’s health or behavior. Until recently, however, it was difficult to scale up this approach. Even with CRISPR, it remained time-consuming to investigate the function of more than a few genes at a time.
That’s changed with a new method developed by biologist Randall Peterson, PhD, dean of University of Utah College of Pharmacy, and H. Joseph Yost, PhD, a professor in the Department of Neurobiology and their colleagues. Their technique, called MIC-Drop, uses DNA as a barcode to label tiny, oil-encased droplets full of CRISPR components targeting different genes. The droplets can be mixed together and then efficiently delivered, one by one, to zebrafish, allowing users to evaluate the functions of hundreds of genes in a single experiment. Zebrafish are already favored by many scientists for genetic studies of biology and disease. MIC-Drop is expected to significantly accelerate their work.
Life-saving antiretroviral medications are vital for tens of millions of people around the world currently living with HIV. Antiretroviral therapy is not a cure, however. HIV infection requires lifelong treatment, and as the use of antiretroviral medications has become more widespread, so too has the emergence of drug-resistant strains of the virus. New treatments are needed to ease the burden of lifelong treatment regimens, overcome drug resistance, and keep HIV under control.
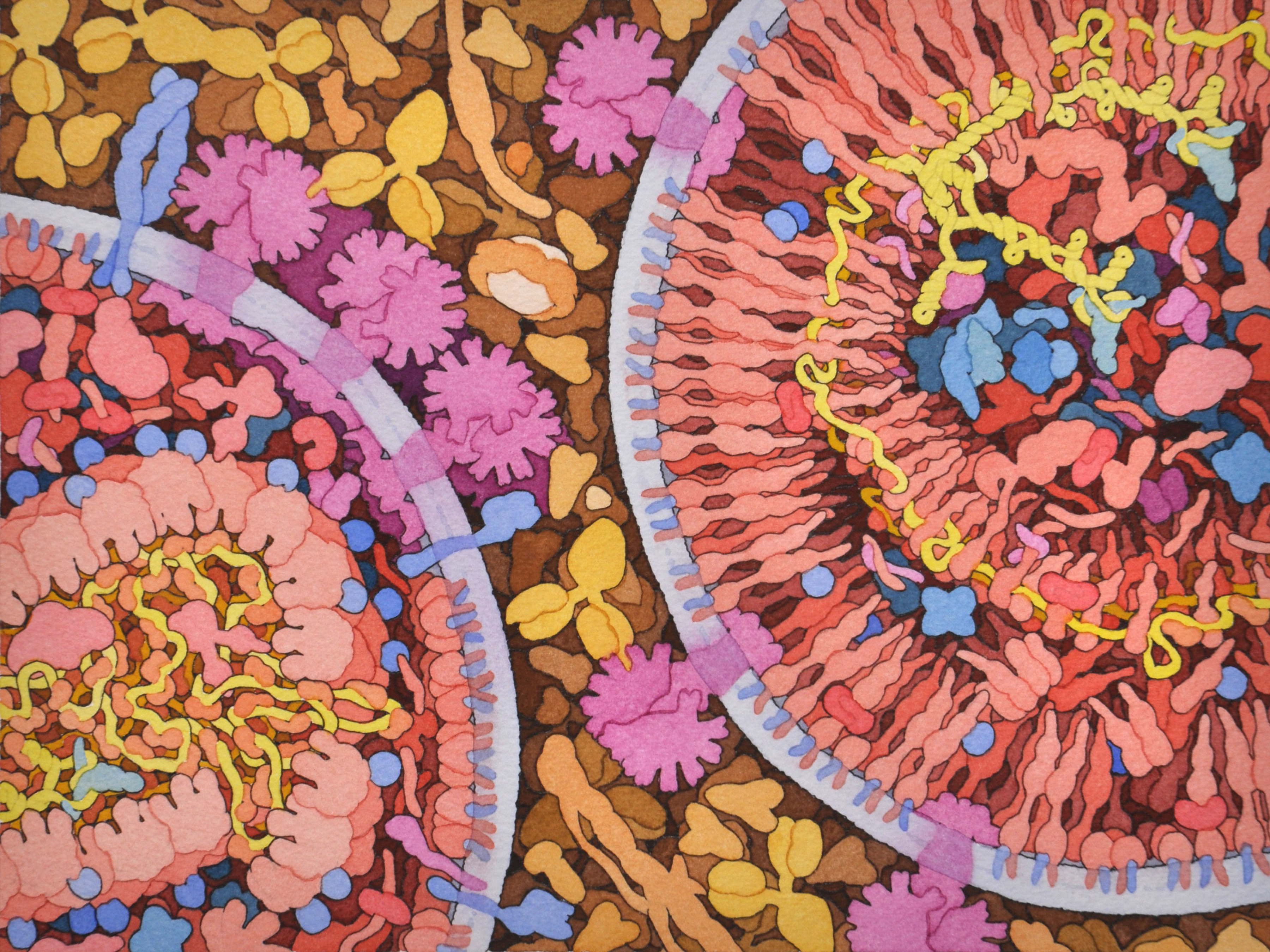
To find novel ways to stop HIV, researchers need to know exactly how the virus enters and infects human cells. Yet despite decades of research, parts of this process remain obscured. Viral replication begins inside the nucleus of an infected cell, where it is difficult for scientists to visualize. To bring the process into better focus, Wesley Sundquist, PhD, professor of biochemistry at U of U Health, Owen Pornillos, a professor at University of Virginia, and colleagues, recreated it in a test tube. Doing so allowed them to see for the first time that the virus needs its inner shell, or capsid, to replicate. Observations like this could help scientists develop new treatments that target the virus. “This is teaching us how HIV infects,” Sundquist says. “We are learning new things about one of the most significant pathogens that humans have ever encountered.”
Most health care providers are now using electronic health records—a technology that has been touted for its ability to bring together many different types of information relevant to a patient’s care and make it easily shareable between providers. It’s clear that electronic health records can serve as comprehensive medical records, tracking patients’ medications, lab results, allergies, imaging, immunizations, and more. But to make the most of that data, clinicians need tools that zero in on the most relevant information and interpret it in ways that can help guide clinical care.
Given the complexity of these datasets, U of U Health scientists working on this problem have turned to artificial intelligence (AI). Applying AI tools to analyze electronic health records from more than 1.6 million patients, Martin Tristani-Firouzi, MD, a pediatric cardiologist, and Mark Yandell, PhD, a professor of human genetics, helped the researchers to identify comorbidities that are most likely to aggravate various cardiovascular conditions and to create risk models that predict clinical outcomes.Their approach could help physicians foresee, prevent, or treat serious heart problems, but its implications may be even broader: “We can turn to AI to help refine the risk for virtually every medical diagnosis,” Tristani-Firouzi says.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist and Alfred Cheung for their work to compile the discoveries and innovations that make this series possible.
Written by: Jennifer Michalowski
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Will Dere, Chris Hill, Amy Tanner