PIONEERING THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health
inspired by nature
February 26, 2021
Inspiration can come from many places—and that’s certainly true for U of U Health scientists following nature’s lead as they create new ways to treat and prevent disease. Seeking better medicines, they look to exotic marine animals, infectious pathogens, and our own immune systems. With chemical precision, they can improve the compounds they find in nature or design better ones, optimizing their function as potential drugs. Their creativity and persistence are not only uncovering prospective therapies for cancer and infectious disease—they are also expanding the ways scientists think about discovering and developing new drugs.
Drug developers are not the first to seek compounds that stop infection, ease pain, or prevent unwanted cell growth. Evolution started solving these problems long ago, and many of today’s drugs are inspired by compounds that plants, animals, and microbes manufacture for their own use.
Medicinal chemist Eric Schmidt, PhD, and colleagues pore through the chemical repertoires of animals and the microbes they live with, seeking natural products with useful properties and investigating how they are made. This work has already led to discovery of an anti-HIV compound in small marine tunicates. By studying the biosynthetic pathways that microbes use to produce drug-like molecules, Schmidt’s team has helped reveal how organisms generate the chemical diversity they rely on for survival. And in bacteria found growing inside reef-dwelling sea creatures, they discovered how a single pathway is capable of synthesizing millions of structurally diverse compounds. Schmidt’s approach to identifying and synthesizing compounds with genetic and chemical tools is revealing new ways to generate and modify potential therapeutics.
Learn more about this discovery.
One abundant source of chemical compounds beneath the sea are animals that have evolved intimate relationships with microbes, relying on them to mediate their relationships with predators, prey, or pathogens. Medicinal chemist and microbiologist Margo Haygood, PhD, and her collaborators have found a rich source of such compounds in shipworms, mollusks that rely on symbiotic bacteria to extract nutrients from their surroundings—usually the submerged wood that they have burrowed inside. Natural products produced by these bacteria could be the starting point for developing potent new antibiotics and other medications.
Dozens of species of shipworms have been found in oceans worldwide, and new ones are still being discovered. In 2017, Haygood was part of an international team that found the first living specimen of a giant shipworm, a species that grows up to five feet long and lives in the mud beneath shallow bays in the Philippines. Bacteria in the giant shipworm’s gills convert hydrogen sulfide gas from the mud into food for the worm. The unusual mollusks and the chemical signals exchanged between them and their bacteria are a reminder of the unexpected diversity that lies hidden in plain sight—and of their exciting potential as a source for chemical discovery.
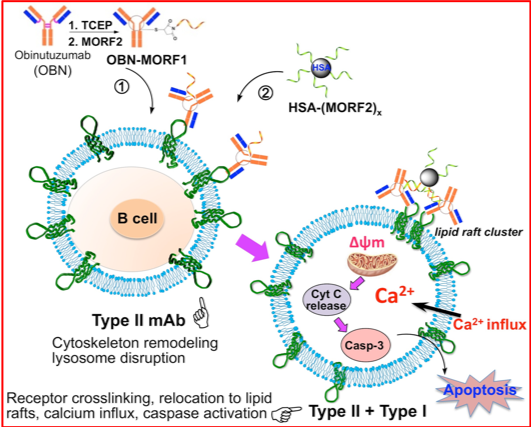
Inspiration for new therapies doesn’t have to come from the depths of the ocean. Other researchers have found strategies for treating disease by looking within. First-line treatments for leukemia and lymphoma often include monoclonal antibodies: lab-generated versions of proteins from our own immune systems that bind to receptors on the surface of cancer cells and help trigger their destruction. These treatments are effective for many patients, but relapse is common. Seeking better options, Utah scientists have designed a new generation of antibody-based therapies that are even more effective at killing their targets.
When antibodies bind to the CD20 receptor on B cells, they can activate those cells’ own cell death pathways. Chemist Jindřich Kopeček, PhD, and his team modified anti-CD20 antibodies to ensure that receptor clustering occurs. The modified antibodies bind first to their targets on a cell’s surface and are then triggered to come together, closely associating with one another and drawing the attached receptors along with them. As the receptors cluster, multiple cell death pathways are activated, killing cells more efficiently than traditional monoclonal antibodies. The findings suggest the modified antibodies might efficiently eliminate cancer cells and improve outcomes for patients.
Finding inspiration for new drugs is one thing. Another challenge is figuring out how to get a medication to the cells where it is needed without causing unwanted side effects. To solve this problem, Utah scientists turned to viruses, which are themselves efficient cellular delivery systems fine-tuned over millions of years of evolution.
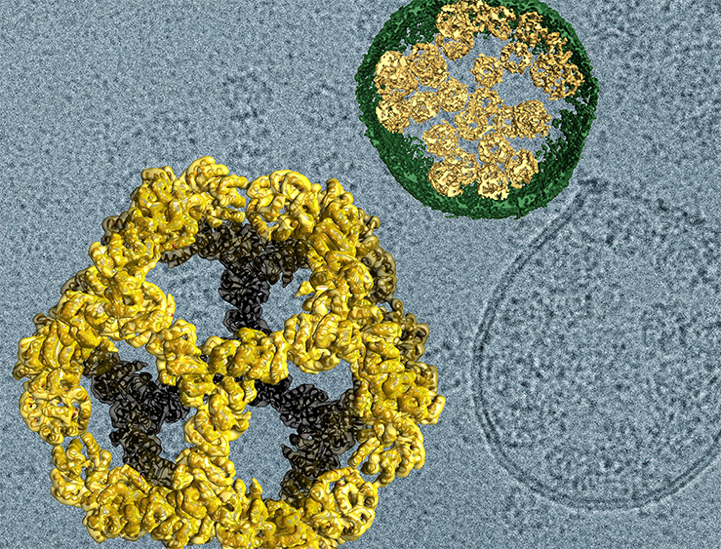
Viruses carry all the tools they need to grab onto cellular membranes and enter cells. Once inside, they deliver genetic cargo that enables new viruses to self-assemble and emerge from the infected cell to begin the cycle again. Mimicking the essential steps in this process, School of Medicine biochemist Wesley Sundquist, PhD, together with Neil King, PhD, at the University of Washington, designed a self-assembling virus-like delivery system that might one day be used to transport therapeutic cargo to target cells. The genetic blueprint they created instructs human cells to generate soccer ball-shaped nanocages, package them inside cell membranes, and export them. After leaving the cell where they originate, the tiny capsules travel to another cell, docking there and emptying their contents upon arrival.