PIONEERING THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health
From Basic discovery to bedside
December 31, 2020
The scientific laboratory is the birthplace of new ideas, rooted in a fundamental understanding of how nature works. While impactful on its own, sometimes discovery is just the beginning. Translating ideas to interventions for improving human health takes added insight and dogged perseverance.
Achieving this milestone is a rarity, but within the past year, three clinical trials have begun testing disease treatments built from basic science discoveries at University of Utah Health. It could take years to determine whether a novel therapy for the deadly amyotrophic lateral syndrome (ALS) and two for a familiar foe, acquired immunodeficiency syndrome (AIDS), could become broadly available. But without a start at the lab bench, these possibilities wouldn’t even exist.
Next-Generation Treatment for ALS

More than 25 years in the making, scientific discoveries led by Stefan Pulst, MD, Dr Med, have reached the milestone of entering human clinical trials with a unique treatment for ALS, or Lou Gehrig’s disease. Patients lose function of nerve cells in their brain and spinal cord, resulting in a life expectancy of five years after diagnosis. The experimental drug is one of a promising new class of next-generation therapies that address the root cause of the disease: the genetic code.
In the 1990s, Pulst couldn’t have known that meeting a family with a different nervous system disorder—ataxia—would set forth a path for the new treatment. He found that the gene ataxin-2 was the source of their ailment. Over decades, he, colleague Daniel Scoles, PhD, and Ionis Pharmaceuticals developed a drug that improved ataxia symptoms in animals by lowering levels of the gene. In a surprise discovery, collaborators at Stanford University revealed that lowering levels of ataxin-2 also dramatically extended the lives of mice with an ALS-like condition. Moving from lab bench to bedside, the ataxin-2 lowering drug is now under investigation as a treatment for people with ALS.
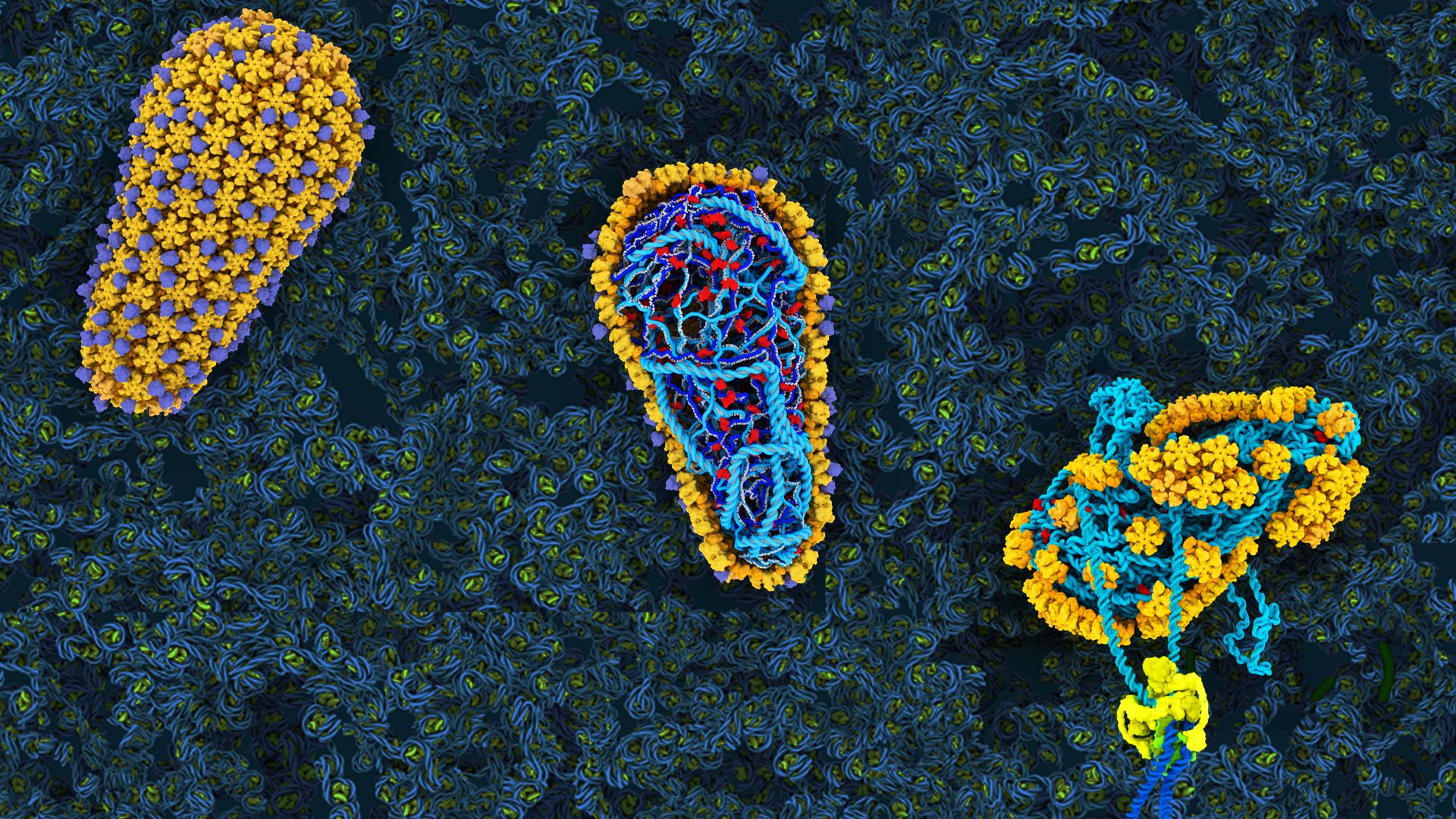
Medications fueled by advances in science and medicine have thankfully converted HIV (human immunodeficiency virus, which causes AIDS) from a death sentence to a chronic disease—at least in countries that have broad access to medicine. Despite these accomplishments, there is still ample room for improvement. Take, for instance, a therapeutic by Gilead that phase 1 clinical trials have shown to be significantly more potent and longer-lasting than current drug “cocktails” that must be taken daily. GSCA1 is the first drug to target the HIV capsid—a shell-like structure encapsulating the virus’ genetic material—inspired by decades of research from leading experts including Wesley Sundquist, PhD, and Christopher Hill, DPhil, both distinguished professors of biochemistry.
Pure curiosity fueled Sundquist’s initial investigations of HIV. In the late 1990s, he became captivated by the first scientific images of tiny cone-shaped capsids and wondered how the virus could build such an intricate structure. His team’s research has since been instrumental in understanding the structures of the 240 hexamer “tiles” that make up the capsid and how they fit together. When it became apparent that the HIV capsid could be a good drug target, Sundquist helped Gilead set up their first drug screens. Most recently, work from his lab has revealed that the capsid is more than a shell—it is instrumental for promoting early steps of infection. Scientists are now poised to dive even deeper into the virus’ biology. How that knowledge might further benefit humankind is only limited by the imagination.
Biochemists Michael Kay, MD, PhD and Debbie Eckert, PhD, have arrived at an alternate experimental AIDS therapeutic by pursuing an entirely different angle. Following Kay’s fascination with synthetic chemistry, his research team has built a drug from the mirror image of pieces of proteins, called D-peptides. The beauty of this approach is that, since D-peptides are not found in nature, the body does not recognize nor degrade them. These qualities could translate to a drug that is long-lasting with minimal side effects.
In collaboration with Navigen, Inc., Kay, Eckert and colleagues developed CPT31, a D-peptide that jams HIV’s infection machinery. It fits into a critical pocket on the virus’ fusion machinery that rarely mutates, blocking the critical process. Research with primates indicate the therapeutic could both prevent new infections and reduce the toll of the disease. Now, the therapy is being tested in the first phase of human trials. Incorporating the best aspects from different therapeutic approaches could make the burden of HIV even more of a distant memory. Kay and Eckert are taking lessons learned even further, applying them toward a crash program for developing drugs that target SARS-CoV-2, the virus that causes COVID-19.