Pioneering the Future
Stories of Discovery & Innovation
Diabetes and Its Complications
August 15, 2022
Diabetes is on the rise around the world, putting hundreds of millions of people at elevated risk of serious problems like blindness, kidney failure, heart attack, and stroke. These complications are all consequences of the body’s inability to properly regulate blood sugar. Chronic overexposure to glucoses damages blood vessels, nerves, and other tissues. But people who use insulin therapy to control their blood sugar are also at increased risk of periods of low blood sugar, which can deprive the brain and other tissues of the energy they need.
The root cause of diabetes is the body’s inability to produce or effectively use insulin. But to treat or prevent these problems, researchers at U of U Health are casting a wider net. They are digging into how diabetes disrupts neural signaling, looking for ways to control leaky blood vessels, and exploring how metabolic imbalances contribute to the disease. As a result, they are identifying new strategies for managing diabetes and improving patient outcomes.
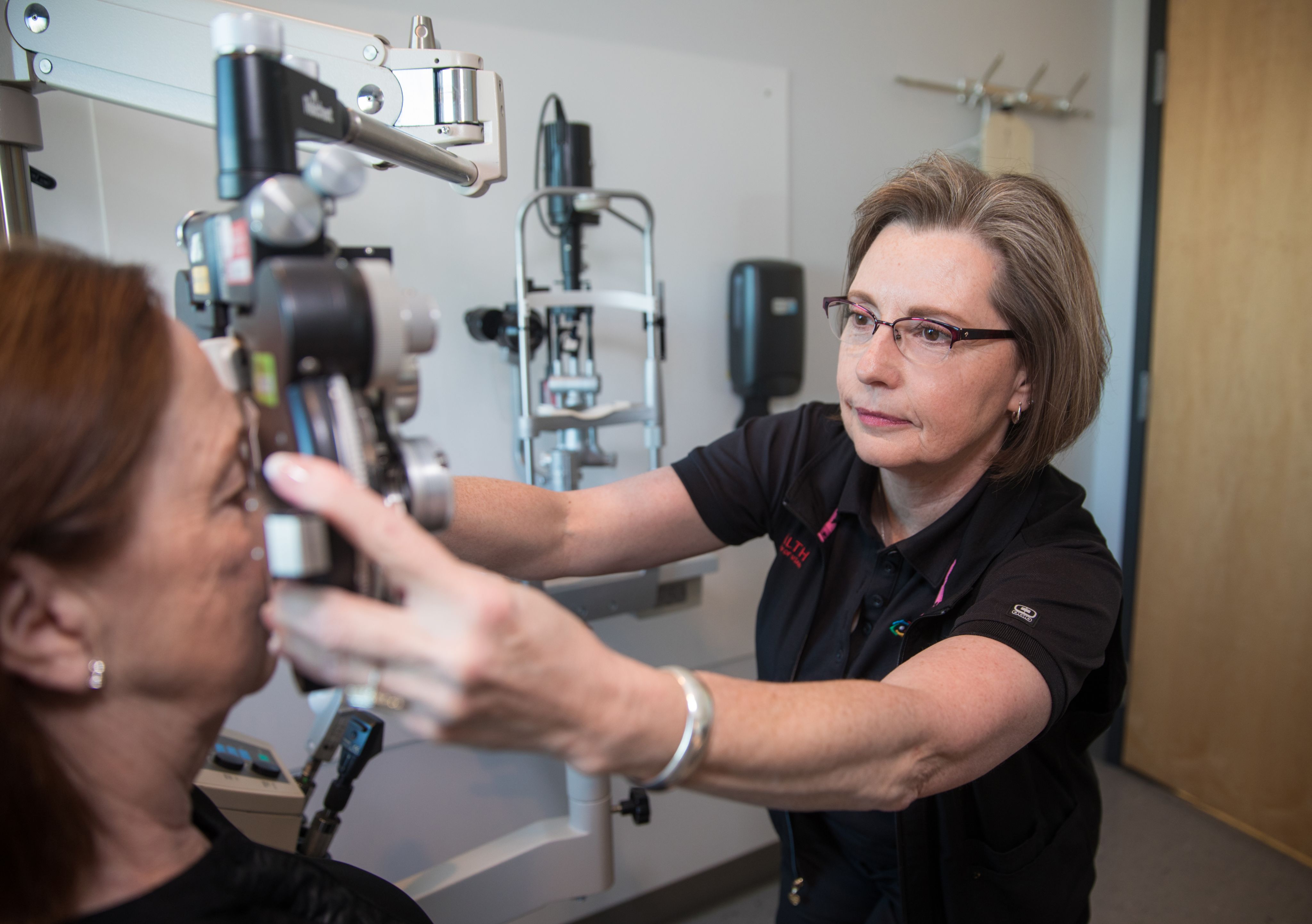
Vision loss is a common complication of diabetes. The most frequent cause is diabetic retinopathy, which occurs when chronic exposure to high blood sugar damages the blood vessels that supply the eye’s retina. The damaged vessels can leak fluid into the retina and blur vision. The problem is exacerbated when the damage triggers the growth of new blood vessels, which tend to be weak and leak easily. In the United States and many other countries, diabetic retinopathy is the leading cause of blindness among working-age people.
Treatments that block VEGF, a signaling protein that promotes blood vessel growth, can reduce the severity of diabetic retinopathy, but they don’t work for everyone. Weiquan Zhu, PhD, and Shannon Odelberg, PhD, from the Department of Internal Medicine, and colleagues discovered that in animal experiments, they can reduce fluid leakage and protect the eyes by blocking a VEGF-regulating protein called ARF6. This approach provides a potential alternative method for treating diabetic retinopathy. The researchers hope that with further development, targeting ARF6 might be an effective strategy for controlling diabetic retinopathy in patients.
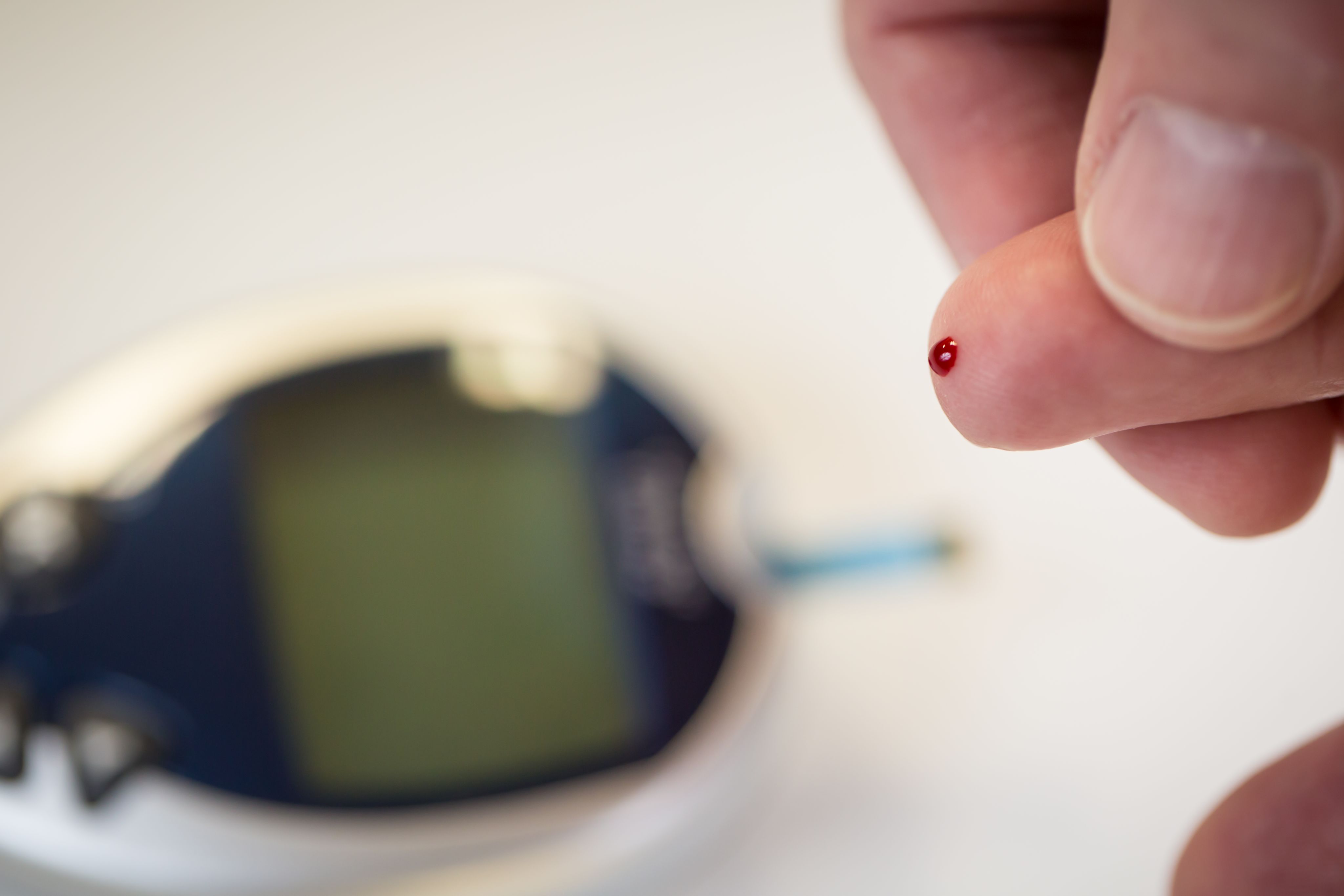
Most people know when their blood sugars dip too low. Symptoms such as dizziness, shaking, hunger or irritability normally prompt them to seek a snack and set things right. But for many people with type 1 diabetes, these signals never come in time. Unless they monitor their blood sugar with a meter, these individuals may not know their blood sugar level is low until the problem becomes severe. Left untreated, low blood sugar (also known as hypoglycemia) can cause confusion, loss of consciousness, seizures, and even death.
Owen Chan, PhD, and Candace Reno, PhD, researchers in the Department of Internal Medicine, and Simon Fisher, MD, PhD, traced hypoglycemia unawareness to signaling problems in the hypothalamus, a region of the brain that both senses and responds to low blood sugar. They discovered that abnormal cellular metabolism interferes with the ability of neurons in this brain region to properly sense a fall in blood sugar levels. This ultimately impairs the release of neurotransmitters that stimulate the secretion of hormones that counter the fall in blood sugars. As a result, the body cannot trigger normal hormonal responses to low blood sugars. The team’s research has led to a clinical trial evaluating whether metoclopramide, a drug that dampens dopamine signaling in the brain, can improve hypoglycemia awareness in people with type 1 diabetes.
Iron is essential to every cell in the body. Without it, cells cannot generate the energy they need for survival. But too much iron can be harmful to cells, so much like blood sugar, its levels must be precisely maintained. When the body’s systems for iron regulation fail and levels get out of balance, one consequence is an increased risk of diabetes.
An excess of iron in the body has long been thought to contribute to the development of diabetes. But Professor of Internal Medicine Elizabeth Leibold, PhD, and colleagues found that too little iron can have a similar effect. Leibold’s lab studies the causes and consequences of iron dysregulation. In their studies of mice genetically engineered to be iron deficient, they discovered that the animals developed an array of health problems, including diabetes. Taking a closer look, they determined that when iron levels are too low, cells in the pancreas cannot produce enough insulin to control blood sugar. Their findings could help researchers develop new strategies to prevent or treat type 2 diabetes.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist and Alfred Cheung for their work to compile the discoveries and innovations that make this series possible.
Written by: Jennifer Michalowski
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Will Dere, Chris Hill, Amy Tanner