PIONEERING
THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health

Demystifying Viruses
January 11, 2022
In 2020, the world’s attention turned abruptly to viruses with the rapid onset of the COVID-19 pandemic. But scientists at U of U Health have long been focused on these diverse and widespread pathogens. Hundreds of viral species infect human cells, employing different tactics to enter cells, manipulate their machinery, and outwit the immune system—all, ultimately, to make more virus. By clarifying how viruses interact with their hosts, research at U of U Health is revealing unexpected aspects of viral biology and pointing toward new strategies for combatting infection.
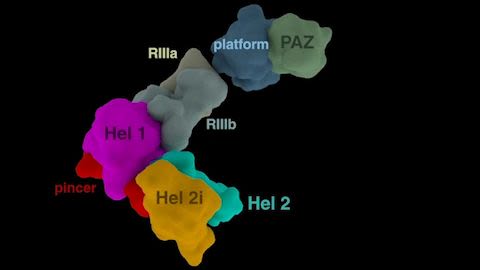
Organisms must be prepared to defend themselves against a wide range of viruses, but it is crucial that their response to intruders does not wreak undue damage on the body’s own cells. A key element of many organisms’ defense strategy is Dicer, an enzyme that chews up double-stranded RNA (dsRNA) made by viruses. It took a series of detailed images of Dicer captured by biochemists Brenda Bass, PhD, Peter Shen, PhD, and colleagues to reveal how this enzyme, which cuts apart double-stranded RNA, works.
Visualizing the enzyme’s structure exposed how it senses a subtle distinction between dsRNA from viruses and double-stranded RNA that belongs to the cell: one has neatly aligned ends, whereas the other’s ends are slightly staggered. Once the enzyme has identified and grabbed onto viral RNA, it reels it in and chops it up. The team made these discoveries by studying a Dicer enzyme from fruit flies; understanding how it works could help researchers develop new ways to control viral infections in people.
Viruses depend on the cells they infect to reproduce, so they have devised a wide range of tricks to take over their hosts’ machinery and create a hospitable environment for themselves. Identifying these strategies can point researchers toward new ways to block infection.
One unusual tactic was discovered in the lab of biochemist Demián Cazalla, PhD. Cazalla’s team found that a herpesvirus that infects non-human primates uses a small piece of RNA to fool its host into slowing down the activity of dozens of genes. The viral RNA attaches to the RNA copies of target genes, then lures in regulatory RNAs produced by the cell to prevent those messenger RNAs from being used. This genetic interference enhances the survival of infected cells and dampens the host’s immune response. It also encourages uncontrolled cell division, leaving infected individuals at risk for cancer. Because this unusual mechanism of host manipulation may also be used by herpesviruses that cause human disease, understanding it could lead to better treatments for patients.
Biological discovery begins with observation. The simple act of seeing can reveal unexpected truths and raise new questions to explore. Viruses are too small to show themselves without extremely powerful imaging tools, and even these capture only blurry, static pictures. But with sophisticated computer animation, a virus’s interactions with its host can be made visible to all.
Merging science and art, biochemist Janet Iwasa, PhD, has captured the life cycle of the human immunodeficiency virus in an exquisitely detailed animation. Viewers watch the virus enter a human immune cell and take over to support its own replication, then see new viruses emerge from the cell, prepared to infect others. Iwasa’s portrayal reflects the deep molecular understanding that scientists have gleaned about these processes through decades of research. The video, an important tool for both research and education, is freely available through the Science of HIV website.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist and Alfred Cheung for their dedicated work compiling research discoveries.
Written by: Jennifer Michalowski
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Will Dere, Chris Hill, Amy Tanner