PIONEERING
THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health
Cardiovascular Science
October 18, 2021
A healthy heart beats about 100,000 times every day, pumping freshly oxygenated blood to every part of the body. At University of Utah Health, researchers are working on several fronts to keep hearts healthy—and to help them recover when they falter. Their work is urgent: Cardiovascular disease is the leading cause of death worldwide, and its prevalence continues to rise. In the United States, one of every four deaths is due to heart disease. With a deeper understanding of both the causes and impacts of cardiovascular disease, U of U Health aims to reduce this burden, both through prevention and better options for treatment.
Mending Broken Hearts
When conditions such as high blood pressure, diabetes, infection, or coronary artery disease increase the burden on the heart, forcing it to work harder to circulate blood, the overtaxed muscle can weaken or become stiff. Eventually, this can lead to heart failure, meaning the heart does not pump blood as efficiently as it should. Most treatments for heart failure aim to relieve patients’ symptoms and prevent the disease from progressing, with a common assumption being that damaged heart muscle cannot be repaired.
In the hopes of developing a new type of therapy, U of U Health scientists have taken a close look at how muscle cells in the heart change during heart failure. Researchers led by Robin M. Shaw, MD, PhD, Director of the Nora Eccles Harrison Cardiovascular Research and Training Institute, have discovered a protein called cardiac BIN1 (cBIN1), which they have found is important for organizing certain structures inside cells. Without cBIN1, the calcium ions whose movements coordinate the heart’s rhythmic contractions can’t get to where they need to be. Patients with heart failure produce less of this protein than people with healthy hearts. Shaw and his team are now exploring the possibility of therapeutically using gene therapy to boost cBIN1 to restore the function of failing heart muscle.
Learn more about this discovery.
Rest and Recover
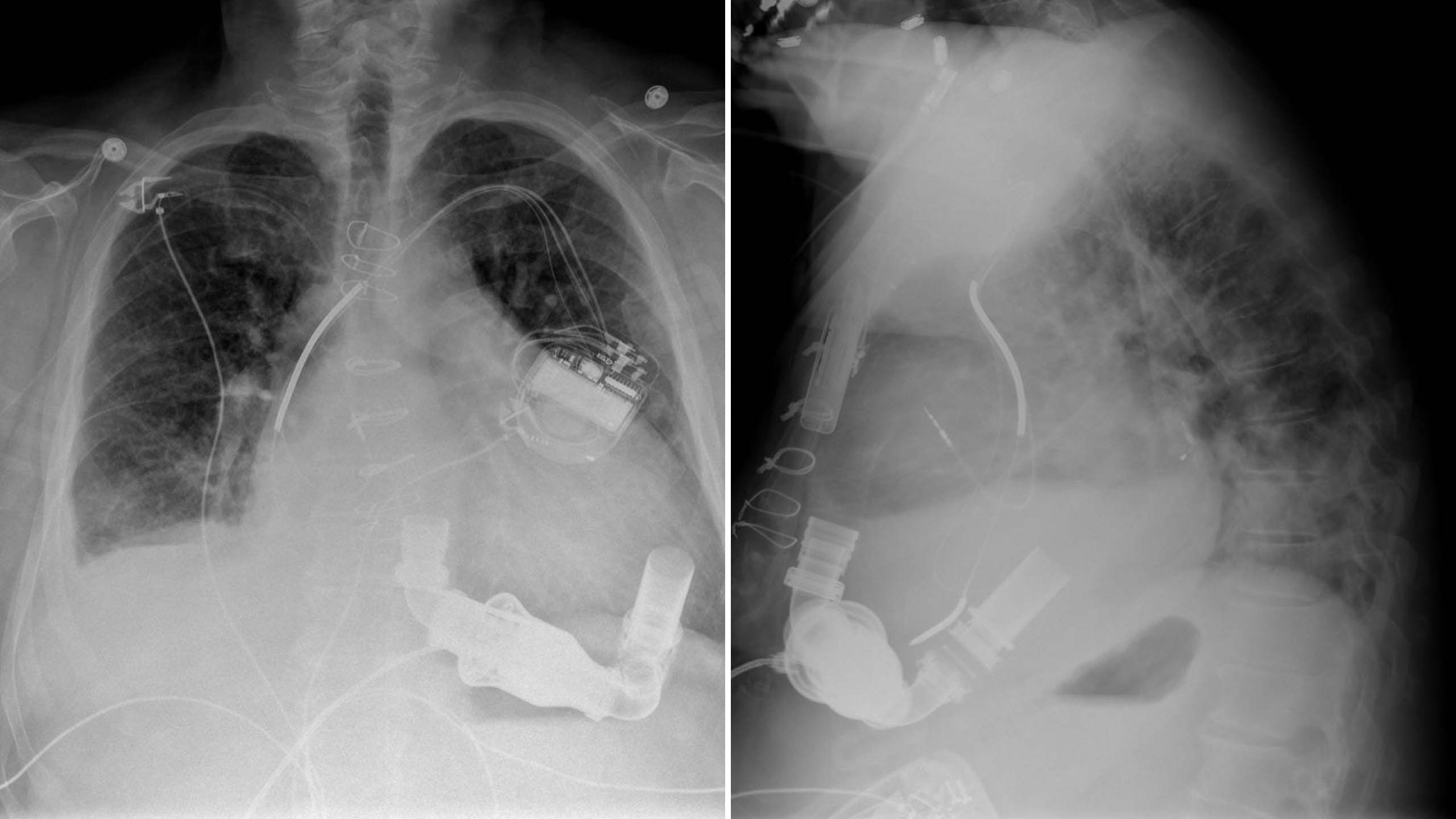
When heart failure is severe, a surgically implanted pump called a left ventricle assist device (LVAD) can work with the heart to keep blood circulating. Most patients who use these devices will need them for the rest of their lives—or until a transplant becomes available. But for some, the assistance provided by a mechanical pump gives a failing heart the chance to rest and recover.
Stavros G. Drakos, MD, PhD, Director of Cardiovascular Research at U of U Health, and colleagues have focused their attention on patients whose damaged hearts recover enough to function on their own after an LVAD is removed. They’ve found metabolic changes within the heart muscle that appear to support structural repair—a discovery that could help researchers design medications that reverse heart damage before an LVAD is needed. They have also tested the effects of giving patients with mechanical pumps standard heart failure medication. In a clinical trial, 19 of 40 patients who received this combined treatment regained enough heart health to live without an LVAD.
Learn more about this discovery.
Personalizing Prevention
Black Americans are 50% more likely to have a stroke and 30% more likely to die from heart disease than white Americans. Both social and biological factors contribute to these disparities, and researchers are still working to understand and address them.
It’s clear that genetics play a role. Paul F. Bray, MD, professor of medicine at U of U Health, has found that Black people are more likely than White people to have blood platelets that are particularly prone to clotting. Platelets form clots at injury sites to prevent excessive bleeding, but wayward clots are the instigators of heart attacks and stroke. Bray and colleagues traced the extra-sticky platelets to a genetic variant that is common among people with African ancestry. What’s more, they found that the variant makes people less responsive to clopidogrel, the drug most commonly used to reduce the risk of heart attack and stroke in people with highly reactive platelets. Their research indicates that an alternative blood thinner, ticagrelor, may be a better choice for affected individuals.
Learn more about this discovery.
Premature Fatigue in Cardiovascular Disease

When the heart is not working efficiently, even mild exertion can be a strain. People living with heart failure may find themselves short of breath after a short walk or routine household chores. Similarly, many people with high blood pressure, whose hearts work extra hard, also tire easily. This fatigue limits people’s ability to participate in the activities they enjoy and makes exercise—an important tool for lowering blood pressure and strengthening the heart—seem daunting or inaccessible.
Researchers have linked this premature fatigue to impaired communication between muscles and the brain. Normally, working muscles signal directly to the brain to alert the body of an increased demand for oxygen and energy. As a result, the heart beats faster, blood pressure increases, and the flow of freshly oxygenated blood to the working muscles increases. Markus Amann, PhD, professor of anesthesiology, and colleagues have found that this circuit is malfunctioning and overreacts to physical activity in people with heart failure or hypertension. This not only causes an exaggerated rise in blood pressure with even just mild provocation, but, importantly, also an abnormally high constriction of blood vessels, limiting the delivery of freshly oxygenated blood to working muscles in the leg or arm. Understanding this effect could help researchers find ways for people with heart disease or hypertension to remain active and exercise safely with less fatigue.
Learn more about this discovery.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist and Alfred Cheung for their dedicated work compiling research discoveries.
Written by: Jennifer Michalowski
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Will Dere, Chris Hill, Amy Tanner

