PIONEERING THE FUTURE
Stories of Discovery & Innovation
at University of Utah Health
Understanding cancer from its beginnings
March 17, 2021
Even the most aggressive tumors begin with a single wayward cell, behaving in defiance of the biological checks and balances that usually limit cellular growth. At Huntsman Cancer Institute, University of Utah Health scientists have created a clearer picture of cancer’s origins by working to understand the genes and molecules that drive cellular growth, both in tumors and in situations where cell division is healthy and appropriate. Their studies have illuminated fundamental processes that maintain tissues and shape developing organisms, as well as how those processes are sometimes misappropriated by cancer cells.
Rapid growth and migration of cells are the hallmarks of cancer, but they are also the processes that shape a developing embryo. These processes share genetic roots, too: many of the genes that play essential roles during development are often found to be misregulated in tumors—reactivating growth signals that should stay reined in after development is complete.
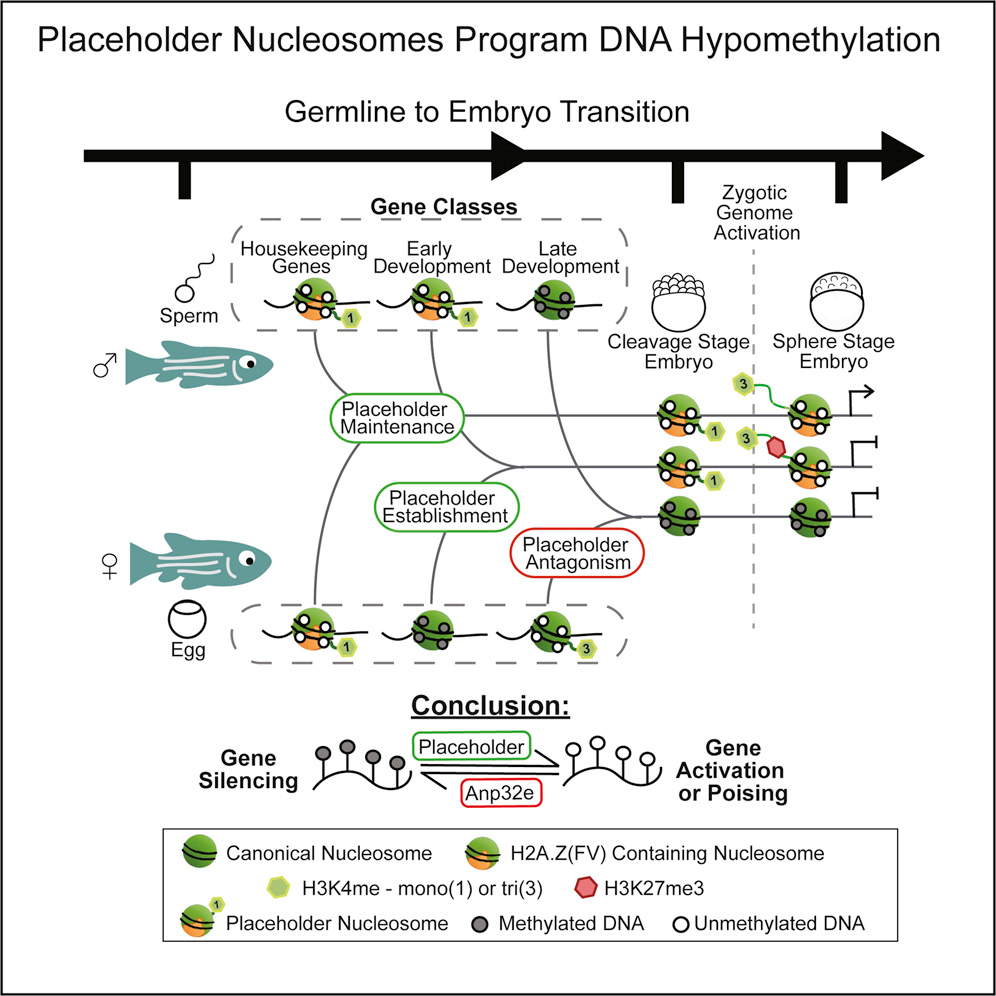
Understanding how genes are used during development can shed light on what happens when those genes go awry. Studies in zebrafish led by Huntsman Cancer Institute Chief Academic Officer, Howard Hughes Medical Institute Investigator, and Professor and Chair of Oncological Sciences, Bradley Cairns, PhD, have revealed how the activation of developmental genes is orchestrated by their packaging. They identified physical structures that occupy specific sites on an embryo’s DNA and serve as platforms that help poise or activate nearby genes. The distribution pattern of these structures is inherited from the parents, and more so the father in certain species. Cairns says understanding these developmental packaging states might help researchers identify precursors of cancer, which in turn could lead to earlier diagnosis of the disease.
Learn more about this discovery.
Although growth generally slows after development, a certain amount of cell division is essential for maintaining healthy tissues in adults, too. In the epithelial tissues that line our organs, blood vessels, and skin, cell death is natural and ongoing, and dying cells must frequently be replaced with new ones to maintain a continuous, protective barrier. Yet too much cell division can lead to cancer, so cell division and cell death must be carefully balanced.
In her lab at the Huntsman Cancer Institute and the Department of Oncological Sciences, Jody Rosenblatt, PhD, found that cells’ ability to detect mechanical tension is critical to maintaining this balance within epithelial tissue. When cells in an epithelial layer become too sparse, stretch-sensitive channels detect the situation and begin dividing more quickly. And when cells become too crowded, the same channels trigger changes that ultimately lead to cell death. When these processes fail, epithelial barriers can become compromised, contributing to diseases like asthma, or overgrown, leading to tumor formation. Rosenblatt’s discoveries reveal an unexpected strategy that healthy cells use to manage their growth.
There is a particularly urgent need to find ways overcome the genetic changes that permit healthy cells to begin growing out of control in the pancreas. The origin site of the third deadliest cancer in the U.S. Pancreatic cancer is often not found until it has spread to other parts of the body and it is mostly untreatable. To figure out how to stop it, cancer researchers are investigating how it starts.
In the Department of Human Genetics, Huntsman Cancer Institute investigator Charles Murtaugh, PhD, and colleagues have traced pancreatic cancer’s origins to cells in the pancreas known as acinar cells. They’ve discovered that a protein called PTF1A normally keeps acinar cells healthy, overriding genetic changes that might otherwise lead to uncontrolled growth. When PTF1A levels drop, however, acinar cells are reprogrammed, reverting to a less mature state in which they can become cancerous. Murtaugh’s team can reverse this process and slow the growth of cancer cells in the lab by reactivating the Ptf1a gene. Based on their findings, they hope it may be possible to treat or prevent pancreatic cancer by developing drugs that ensure cells have a healthy supply of PTF1A.
Aggressive tumors often find ways to resist the drugs that are used to combat them, causing patients to relapse after successful treatment. To overcome or prevent drug resistance, researchers need the kind of deep understanding of a drug’s target that is being generated by Huntsman Cancer Institute investigator and Assistant Professor of Oncological Sciences, Benjamin Myers, PhD.
In their studies of a cancer-promoting protein called Smoothened, Myers and colleagues uncovered a molecular interaction that could be that protein’s Achilles heel. Blocking Smoothened, which helps drive cell growth both in developing embryos and in tumors, can stop cancer’s spread, but eventually tumors adapt and the approach becomes ineffective.
To activate a cell’s growth pathways, Smoothened must interact with a cholesterol molecule. Structural studies by Myers’ team revealed that Smoothened’s cholesterol-binding site resides deep within the portion of the protein that spans a cell’s membrane. They think preventing cholesterol from nestling into this pocket might be a key to developing anti-Smoothened treatments that tumors cannot circumvent.