PIONEERING THE FUTURE
Stories of Discovery & Innovation

Advancing Translational Science
April 17, 2023
Medical advances often begin in the lab, emerging from a deeper understanding of the biological processes that underlie health and disease. Biological discovery creates opportunities for new treatments and prevention strategies, but it takes vision and commitment to recognize those opportunities and translate the new knowledge into real improvements to human health.
U of U Health scientists’ translational research addresses a broad range of human health challenges, from aging hearts and autoimmune disease to joint injuries that hasten the onset of arthritis. Building on what they have learned in their labs, these researchers are exploring new treatment strategies with an eye toward bringing better options to patients.
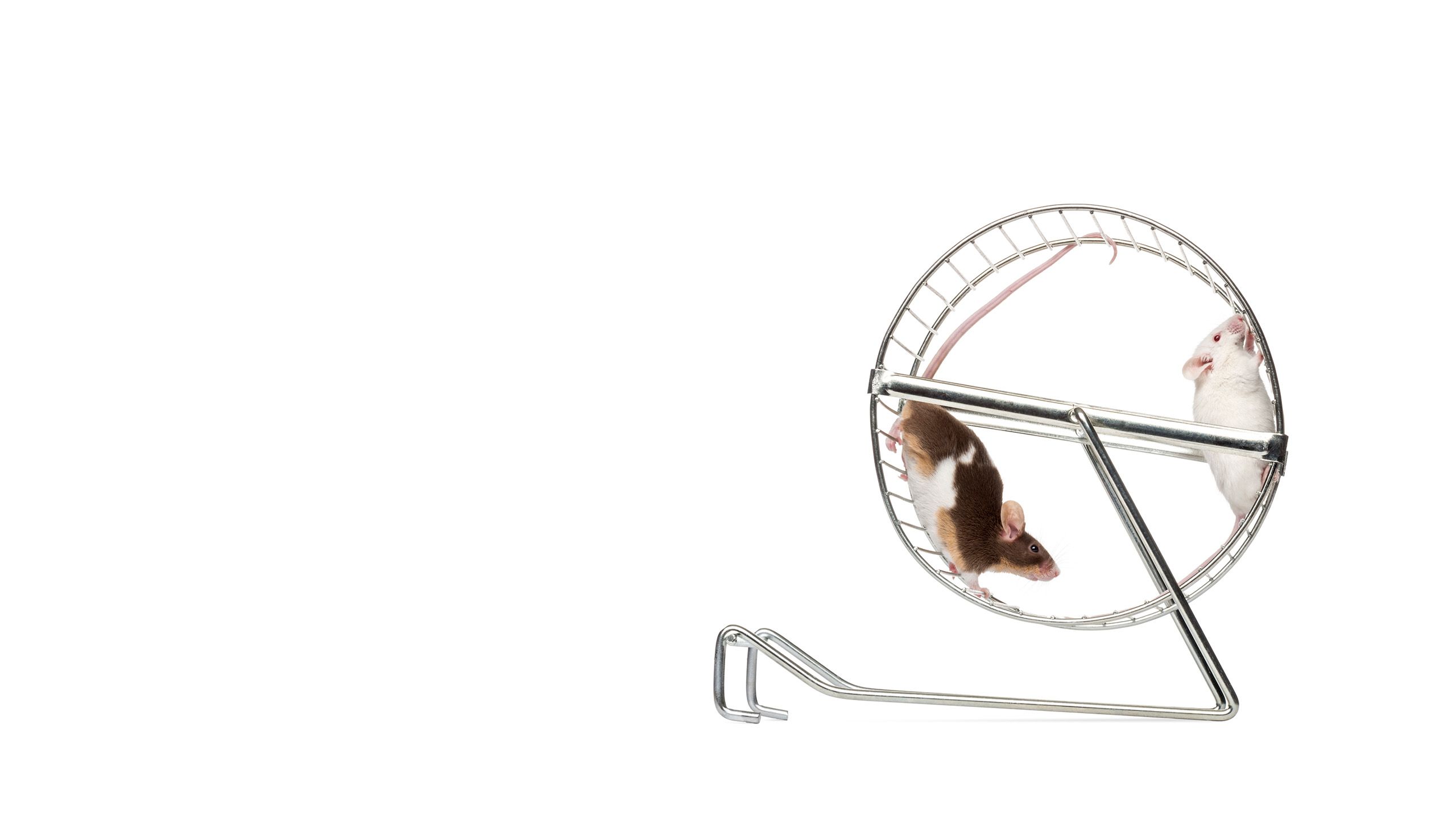
The cells of the heart work together tirelessly to pump blood throughout the body. One key to keeping those cells working efficiently is a process called autophagy—a cellular recycling program that clears away damaged cellular components and misshapen proteins. But autophagy slows as we age, and debris can begin to build up inside cells. This can make it harder for the heart muscle to pump blood, increasing the risk of heart failure.
College of Health researchers J. David Symons, PhD, and Sihem Boudina, PhD, along with graduate student Jaemin Cho, have found in animal experiments that autophagy can be restored late in life with regular exercise. When very old mice ran on a treadmill daily for three months, their heart cells did a better job of ridding themselves of damaged components than the heart cells of sedentary mice. More importantly, the revived cellular recycling was associated with improved heart function. The researchers’ findings offer evidence that exercising late in life helps keep the heart’s cells healthy.
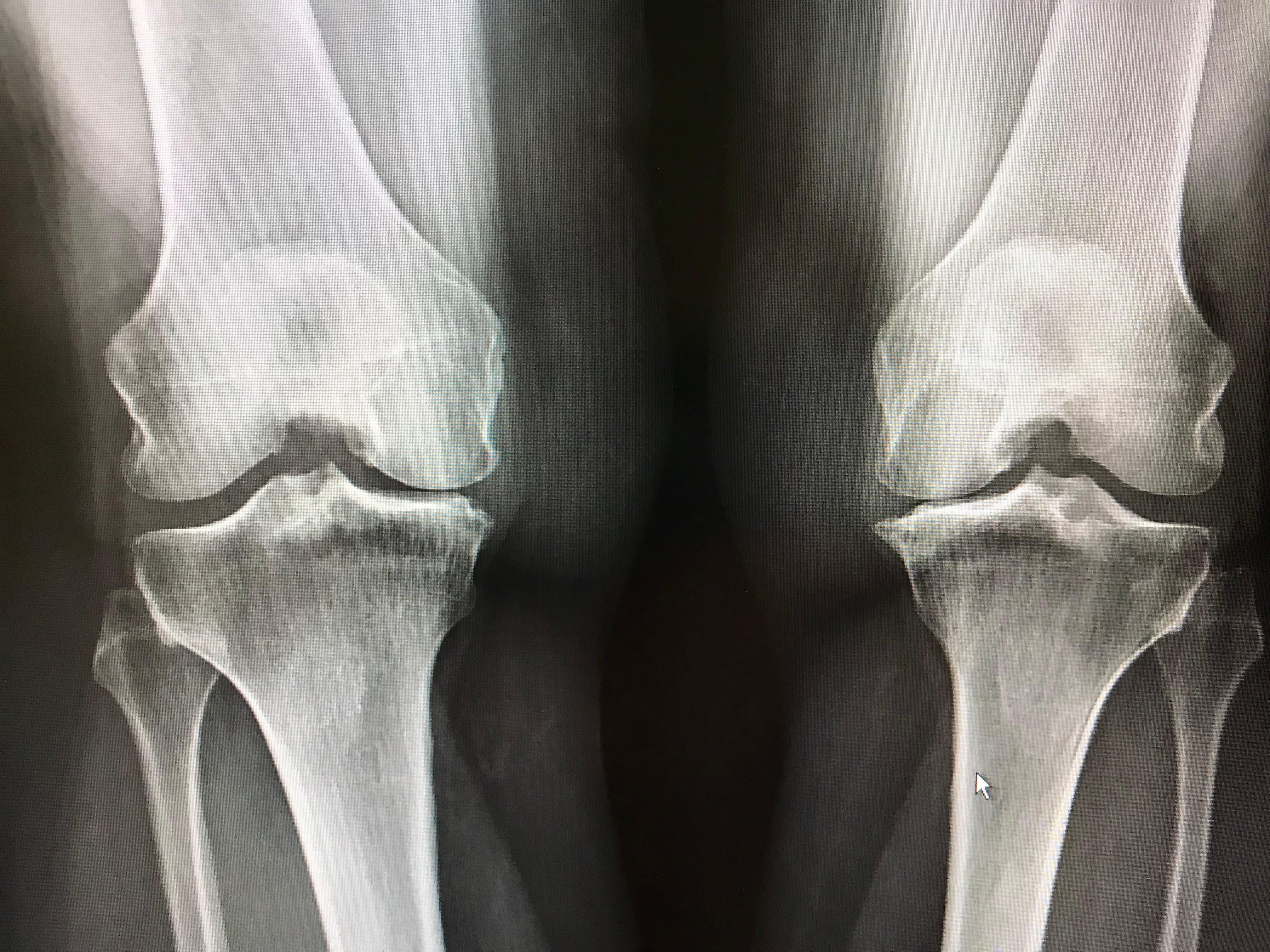
Damage to joint-cushioning cartilage in a knee or other joint can cause significant swelling and pain. These injuries can be particularly frustrating because damaged cartilage usually does not heal on its own. Surgery may be needed to repair the damage and promote healing. Untreated, the injury can lead to further deterioration in the joint and accelerate the development of arthritis.
While fully developed joints don’t produce much new cartilage, cartilage-generating cells are active earlier in life. College of Pharmacy researcher Makoto Kondo, PhD, and colleagues took some of these fast-growing juvenile cells, called chondrocytes, and coaxed them to assemble into sheets of tissue that can be used to replace or repair damaged cartilage. When they transplanted the sheets into rats with cartilage damage, the cartilage quickly regenerated and the animals regained the ability to bear weight on their injured leg. Kondo is hopeful that juvenile chondrocyte sheets—engineered from cells that would otherwise be discarded during certain pediatric surgeries—could become an accessible and cost-efficient material for cartilage restoration in patients.
Millions of people worldwide have autoimmune conditions that cause their immune systems to attack their own tissues. Many take medication to keep their wayward immune systems in check. Such drugs usually work by dampening the overall activity of the immune system. This protects the body’s healthy tissues from autoimmune attack, but it also leaves people more vulnerable to infection.
U of U Health scientists have found a way to target the errant immune cells that cause some autoimmune conditions, including type 1 diabetes and multiple sclerosis. Those cells carry a tell-tale signal of their dangerous behavior: a protein on their surface called PD-1. Mingnan Chen, PhD, and colleagues in the Department of Molecular Pharmaceutics developed a toxin that kills immune cells with the PD-1 marker without disturbing other immune cells. The immunotoxin had dramatic effects when the team tested it in mice: animals paralyzed by a multiple sclerosis-like condition regained the ability to walk, and the onset of diabetes was delayed. Peng Zhao, PhD, a former graduate student in Chen's lab, says a similar strategy might be deployed to treat autoimmune conditions more safely in patients. "Using this method, we may avoid long-term immune deficiency caused by common treatments for autoimmune disease," she says.

Pioneering the Future: Stories of Discovery & Innovation at University of Utah Health
Special thanks to Wes Sundquist and Alfred Cheung for their work to compile the discoveries and innovations that make this series possible.
Written by: Jennifer Michalowski
Editing by: Julie Kiefer
Layout and Design by: Kyle Wheeler
Production Supervision: Abby Rooney, Julie Kiefer, Kyle Wheeler
Supported by: Rachel Hess, Chris Hill, Amy Tanner