A Change of Heart
Forty years after Barney Clark received the world’s first permanent artificial heart, his legacy continues to inspire research into treating cardiovascular disease.
By Stephen Dark
December 20, 2022
A Change of Heart
Forty years after Barney Clark received the world’s first permanent artificial heart, his legacy continues to inspire research into treating cardiovascular disease.
By Stephen Dark
December 20, 2022
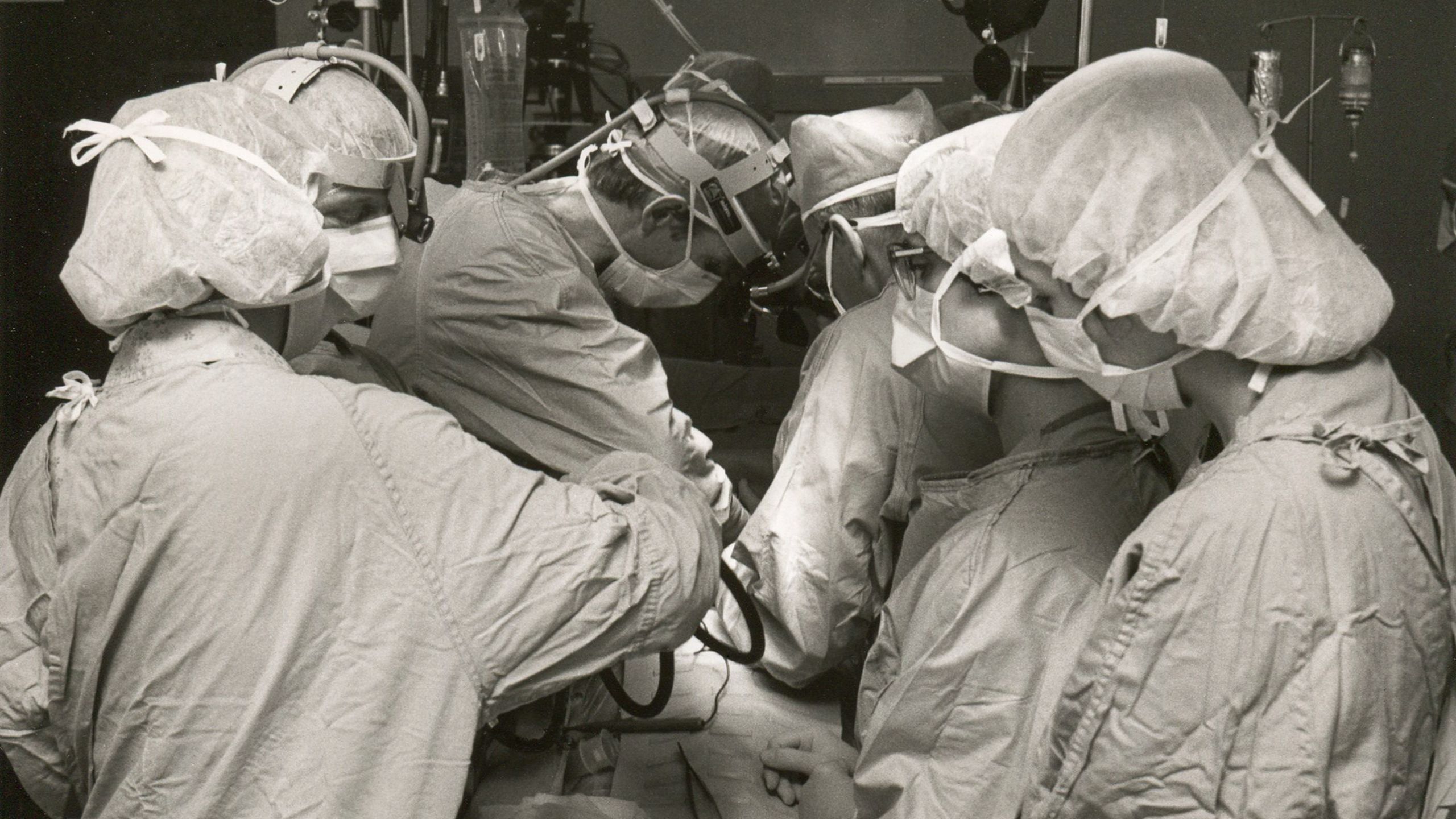
William DeVries, MD, was at a University of Utah sports complex unwinding after work with cardiologist Jeffrey L. Anderson, MD, when he revealed a bombshell.
After seven months of negotiations through early 1982, the Food and Drug Administration had given approval for a team of surgeons and support staff at University of Utah Health to conduct the first permanent implantation of what’s called a total artificial heart in a human being. Until then, total artificial hearts had only been used as a mechanical stand-in for a working heart until a donor heart became available.
The only problem was that DeVries, a cardiothoracic surgeon, didn’t have a patient who met the requirements for this revolutionary advance. That person needed to be in severe heart failure, over 60 with strong family support, and without any other medical options.
Anderson suggested his patient, Barney Clark, DDS, a 61-year-old Utah-born, Seattle-based dentist. The war veteran had a loving family and was in class IV heart failure, entering a terminal stage of the disease. Initially, Clark was not keen. When he watched DeVries, at the surgeon’s invitation, implant the latest University of Utah Health iteration of its total artificial heart, the Jarvik-7, in a cow and saw how it woke up and walked around, he declined to take part. “I have some problems that this cow doesn’t have—it’s healthy going into this, I’m not.”
But when his son had to carry his too-sick-to-walk father to the Thanksgiving table, Clark changed his mind. He wanted to “leave a small mark in the world,” he told his wife, and admittedly it would be “nice to brush my teeth and comb my hair without being exhausted.” Fundamental to his decision was a desire to give back.
“I’ve been kept alive for the last four years through all kinds of medicine and therapies,” he said, that people before him had given their lives for so that he might benefit from them. “Now, it is my time to pay them back.”
After reading 11 pages of disclaimers, Clark signed off on having his ailing heart removed and the 400-pound, air compressor-powered Jarvik-7 put in its place. Jarvik-7 was named by Willem Kolff, MD, PhD, founder and leader of U of U Health’s total artificial heart project, after Robert Jarvik, MD, one of the key team members.

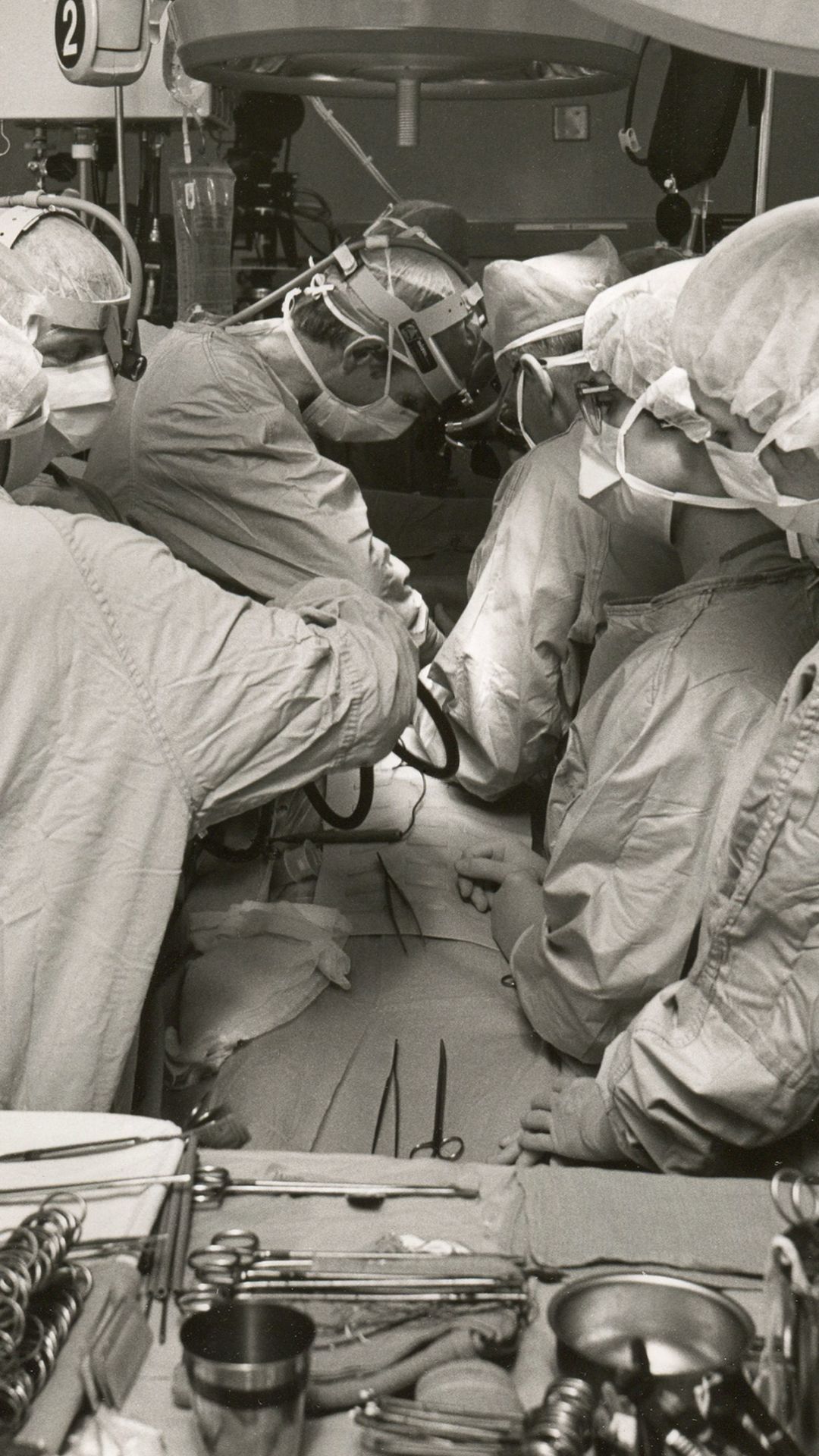
The UTAHDRIVE console system was used with the first permanent artificial heart patient, Barney Clark, DDS. It contains primary and backup systems with manual controls for controlling the patient’s heart, as well as important measuring and recording devices.
The UTAHDRIVE console system was used with the first permanent artificial heart patient, Barney Clark, DDS. It contains primary and backup systems with manual controls for controlling the patient’s heart, as well as important measuring and recording devices.
On December 2, Clark’s already poor health spiraled into critical condition, his blood pressure a dire 80 over 40. As he was rushed into the OR, Clark told his wife, Una Loy, “Honey, in case I don’t see you again, I want you to know you’ve been a darned good wife.” And so began the story of an evolutionary landmark in the race to respond to the Institute of Medicine’s clarion call in the mid-1960s for a total artificial heart.

William C. DeVries, MD, with Jarvik-7 and heart model.
William C. DeVries, MD, with Jarvik-7 and heart model.
The story of Clark revealed the astonishing new measures that man could now take to prolong life when the organ at its center was failing. This display of bravery by one patient and his family stepping into the arena of medical experimentation in the name of progress would hypnotize the world for three months.
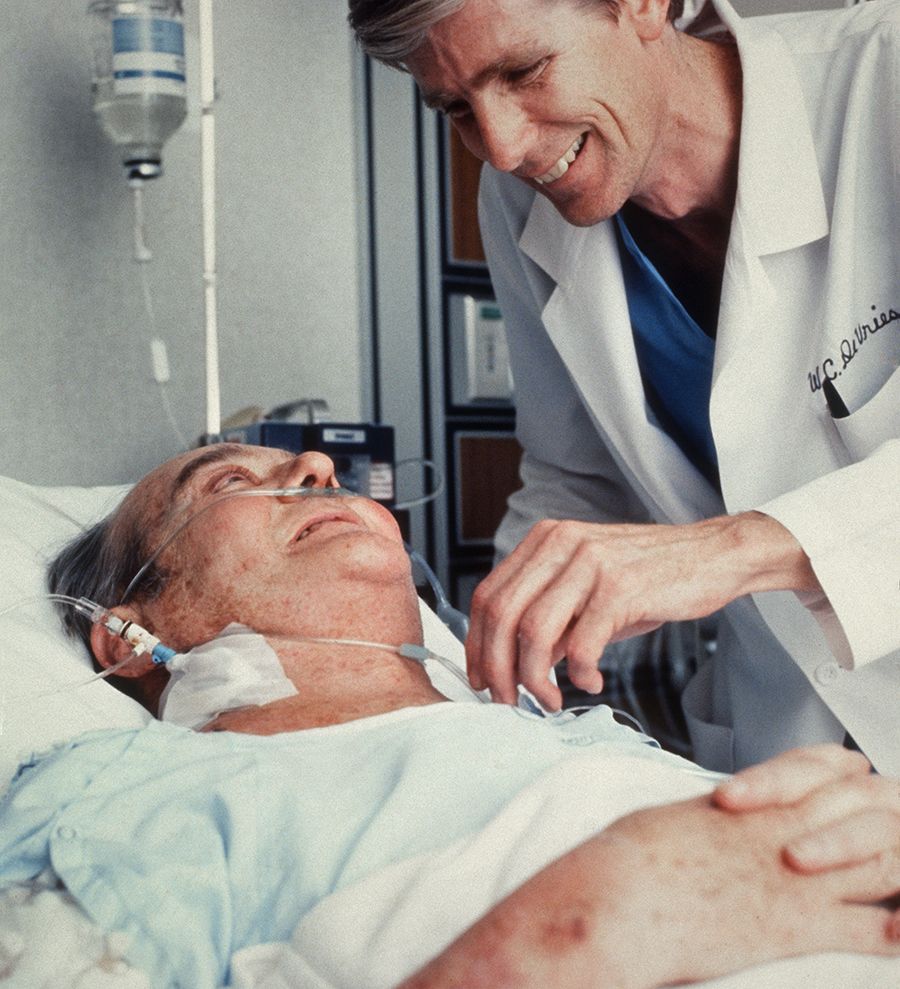
William C. DeVries, MD, tends to patient Barney Clark, DDS.
William C. DeVries, MD, tends to patient Barney Clark, DDS.
From that very first overnight cliffhanger, when a near-death Clark was wheeled into the OR, 350 journalists camped out in the U’s cafeteria, hung on every word and pronouncement shared by Clark’s surgeons, cardiologists, nurses, psychiatrist, and family. Would this saga be a tantalizing story of medical promise for the 600,000 Americans likely to die of heart disease each year—or of tragedy and dashed hopes?

Chase N. Peterson, MD, (left) 11th president of the University of Utah, talking at a press conference regarding the artificial heart surgery. He is with cardiac surgeon Lyle D. Joyce, MD, PhD, (middle) who implanted the artificial heart with William C. DeVries, MD (right).
Chase N. Peterson, MD, (left) 11th president of the University of Utah, talking at a press conference regarding the artificial heart surgery. He is with cardiac surgeon Lyle D. Joyce, MD, PhD, (middle) who implanted the artificial heart with William C. DeVries, MD (right).
Around 9:15 pm, DeVries made an 18-inch incision in Clark’s chest, sawed through the breast bone, then opened the rib cage to reveal the weak, distended heart almost double the normal size that the plastic and aluminum total artificial heart would replace. Once DeVries removed the left and right ventricles, there was no going back.
The team stepped back from Clark seven hours later at 4:15 am. For all of them, it had been an emotionally intense, even spiritual, experience.


It would be three hours before Clark opened his eyes. For the first time in a long time, he felt his own heartbeat, albeit with the blood pressure of an 18-year-old, 119 over 75.
“I want to tell you,” he said to Una at his bedside, “even though I have no heart, I still love you.”
Clark lived for 112 days, eventually succumbing to complications that led to multiple organ failure. DeVries talked with Una and agreed that it was time to turn off his still perfectly functioning artificial heart.
Although doctors rarely go to patients’ funerals, in Clark’s case, he had asked them to attend, and they did. DeVries said Clark was “a remarkable man, a pioneer to match these western lands.”
From Bench to Bedside
Forty years on from that extraordinary saga, cardiovascular disease remains not only the number-one cause of death and disability in the United States—but also “the biggest unsolved mystery in cardiology today.”
“Indeed, we remain in the medical equivalent of the Stone Age of treating heart failure,” said Robin Shaw, MD, PhD, director of the Nora Eccles Harrison Cardiovascular Research and Training Institute (CVRTI).
That’s because heart research historically focused on vascular biology—with clear results. Deaths from heart attacks, which are blockages of the arteries of the heart, are a quarter of what they were decades ago. At present, damaged hearts can survive arterial blockages, thanks to drugs, stents, and bypass surgery. Yet often the patients then develop heart failure.
More than 6,000,000 Americans live with chronic congestive heart failure, which is the inability of the heart to effectively pump blood around the body. There are 800,000 new diagnoses of heart failure each year in the United States, and it contributes to the death of 400,000 Americans annually. Heart failure-related hospitalization is the single highest health care cost to Medicare. For patients living with the serious condition, it means a significantly reduced quality of life. In advanced stages, patients’ chronic state of breathlessness all but immobilizes them. While around 3,500 heart transplants are performed in the U.S. each year, that barely even begins to address the sheer number of those in heart failure praying for a healthy heart.
Which is where Barney Clark’s legacy comes in.
Behind the gift of each day that Clark got to live after his life-changing operation lay 20 years of driven, innovative work. The roots of the Jarvik-7 artificial heart trace back to Kolff, a Dutch immigrant, inventor, and worldwide pioneer, who joined the University of Utah in 1967 to run its artificial organs division at the Institute of Biomedical Engineering.
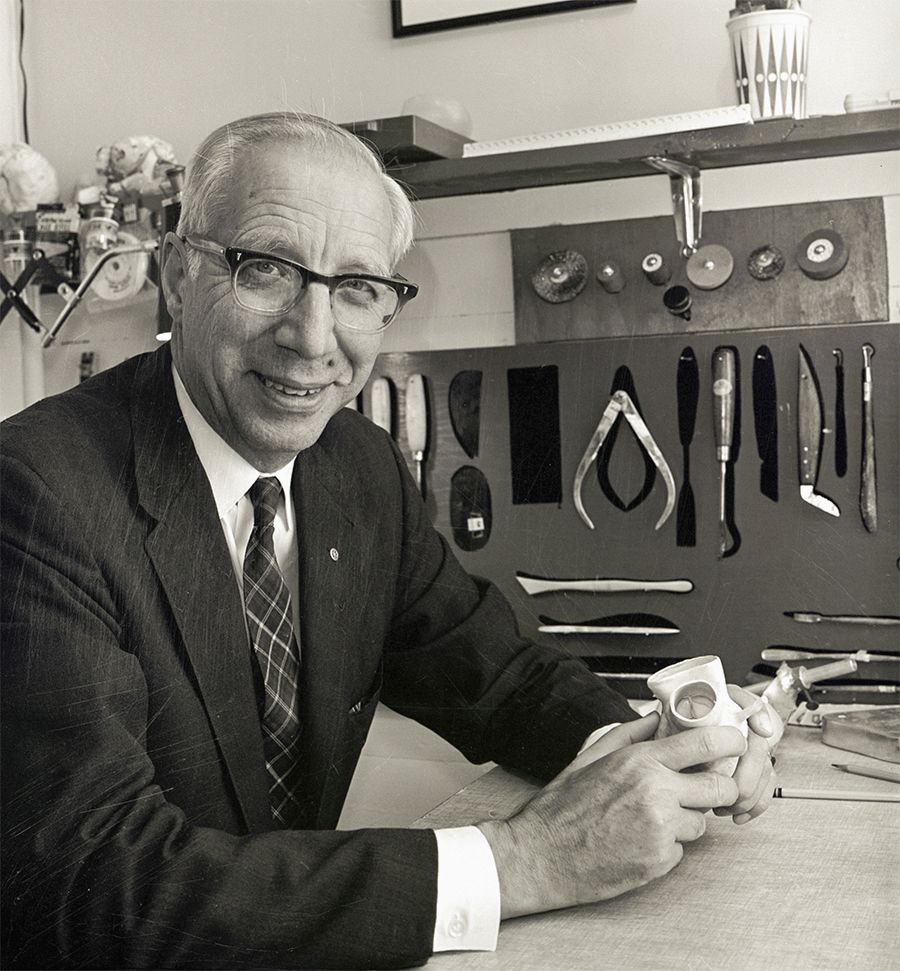
Willem J. Kolff, MD, PhD, the godfather of artificial organs and the inventor of the artificial heart.
Willem J. Kolff, MD, PhD, the godfather of artificial organs and the inventor of the artificial heart.
His team, which included many investigators, surgeons, and physician-scientists, developed and tested numerous iterations of what’s known as the Utah Total Artificial Heart, leading up to the Jarvik-7 that beat in Clark’s chest.
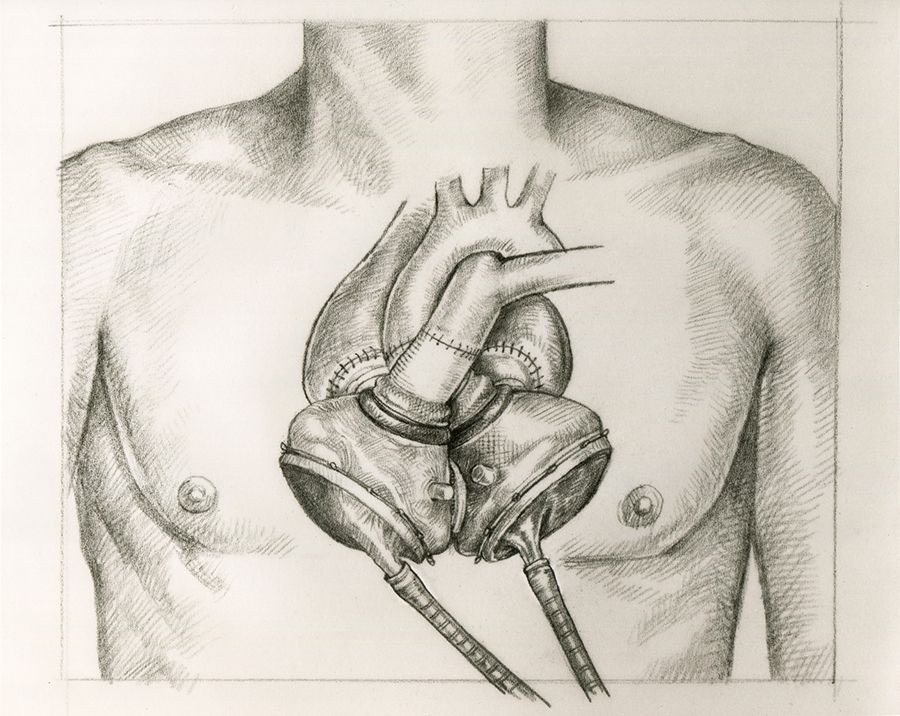
Diagram of the Jarvik-7 artificial heart.
Diagram of the Jarvik-7 artificial heart.
In the decades since, the innovative cardiac legacy of the world’s first total artificial heart, combined with Clark’s gift at the end of his life, has continued to fuel advances in cardiovascular science and medicine at U of U Health.
Today, heart research is nucleated at the Nora Eccles Harrison Cardiovascular Research and Training Institute (CVRTI), which is located on the health sciences campus, a minute’s walk away from the hospital. Designated as an institute in 1969, CVRTI has been home to a multitude of multidisciplinary investigators, both laboratory scientists and physician-scientists, dedicated to bench-to-bedside research into cardiovascular disease and education.
Clark’s decision to say “yes” to the operation, Shaw said, was the result of an intense bench-to-bedside research effort, demonstrating that, at U of U Health, “We can do cutting-edge science, advance it to organ-level pre-clinical studies, and then bring it to patients.”
With CVRTI opening a new wing to expand its laboratories to 20 in total, Shaw said, its national reputation as the largest freestanding collection of researchers in muscle biology, metabolism, and electrophysiology would continue.
“We have all the personnel—and are still working on bringing in more—to do not just basic science but the translational implementation and bring it to the bedside,” Shaw said. “Essentially, from soup to nuts. I truly believe the Jarvik-7 heart is the model of how that can be done.”
Inspired in large part by Clark’s milestone procedure, CVRTI has been at the forefront of groundbreaking new approaches to cardiovascular disease. The accomplishment is made possible by interdisciplinary collaboration across three colleges and six departments at U of U Health.
The institute’s innovations have included identifying the genetic and acquired origins of long QT syndrome, which leads to sudden cardiac death; mapping heart electrical activity, a technique that laid the groundwork for non-invasive cardiac ablation to treat dangerously irregular heartbeats; understanding how left ventricular assist devices (LVADs) can promote myocardial recovery and use this to develop new drug therapies for heart failure; and now working toward developing a gene therapy—made possible by the recent discovery of the cardiac BIN1 protein—that may drastically improve options for patients with chronic heart failure.
As Stavros Drakos, MD, PhD, a physician-scientist at CVRTI, medical director of the clinical LVAD program, and professor of cardiology at the Spencer Fox Eccles School of Medicine at the University of Utah, said, “We are invested in figuring out how to help a failing heart recover its function with just a little help from us.”
The Source of All Wisdom
While the total artificial heart remained an important option for a minority of patients over the next 40 years, with 306 implanted so far, the Clark legacy did more than that. It brought unstoppable energy to the long-term work at CVRTI and U of U Health, Shaw said.
In the 1990s, the institutions were a leading force in research into the molecular basis of cardiac arrhythmia.
Michael Sanguinetti, PhD, Mark Keating, MD, and clinical coordinator Katherine Timothy discovered the first human mutation that leads to inherited long QT syndrome, a cardiac disorder that can result in sudden cardiac death. Keating joined the Eccles Institute of Human Genetics at U of U Health in 1989, while Sanguinetti, a cellular electrophysiologist who worked on discovering novel antiarrhythmic agents, joined in 1993. They’d run and mountain bike in the foothills behind the university campus, discussing their ideas. Sanguinetti later moved to CVRTI and continued his impactful research program there.
Yoram Rudy, PhD, a cardiac bioelectricity and arrhythmia guru, developed his theory of how the heart’s electricity worked while he was a visiting scientist at CVRTI in the 1990s. Rudy was a mentor and teacher of CVRTI director Shaw, who regards Rudy “as the source of all wisdom in cardiac electrical theory.”
CVRTI continued to attract powerful minds in the 2000s, including two who have become key figures in investigating as well as treating the failing heart.
One was Drakos, whom Shaw called “the most prominent individual in this country right now on the concept of myocardial recovery.” The other is Craig Selzman, MD, chief of the Division of Cardiothoracic Surgery, who is now a leader in advancing cardiac care in the region.
Technology Improves Biology
In the years after the first in-human, long-term use of the total artificial heart, scientists across the country made improvements in technology that led to the more widespread use of a partial artificial heart.
The left ventricular assist device (LVAD) is a heart pump that helps the failing organ work better rather than replacing it entirely.
This advance, and its potential, captured the imagination of Drakos and Selzman early in their careers, from opposite sides of the world. Today, the two physician-scientists are leading efforts in leveraging the LVAD in completely new ways to improve patients’ prospects.
After Drakos finished his MD/PhD in Athens, Greece, in 2001, the former chief of cardiology at the University of Athens summoned him to the University of Utah. For decades, U of U Health had served as a training ground for Athens’ cardiologists, and Drakos followed in the esteemed footsteps of the many who had come before him. For two years, Drakos learned how to manage the complex care of patients living with LVADs so he could bring that training and heart failure therapeutic option back to Greece and Europe.

Stavros Drakos, MD, PhD, (right) and his wife Aliki Milioti-Drakos (left) standing at the Acropolis in Athens during Stavros’ time as a medical student at the University of Athens School of Medicine.
Stavros Drakos, MD, PhD, (right) and his wife Aliki Milioti-Drakos (left) standing at the Acropolis in Athens during Stavros’ time as a medical student at the University of Athens School of Medicine.
When he saw how LVADs were supporting “sick-like-a-dog patients that would have been dead otherwise, I was shocked.” Within a handful of years, U of U Health asked Drakos to return and join its faculty.
Meanwhile, Selzman pursued medicine as a student at Baylor College of Medicine in Houston, where one of the world’s greatest heart surgeons, Michael E DeBakey, MD, pioneered operations in the 1950s and implanted the world’s first LVAD in 1966. During Selzman’s first year at U of U Health in 2008, there was only one LVAD patient, he recalled, and that man could not leave the hospital because the pump was extracorporeal—it sat outside the body.

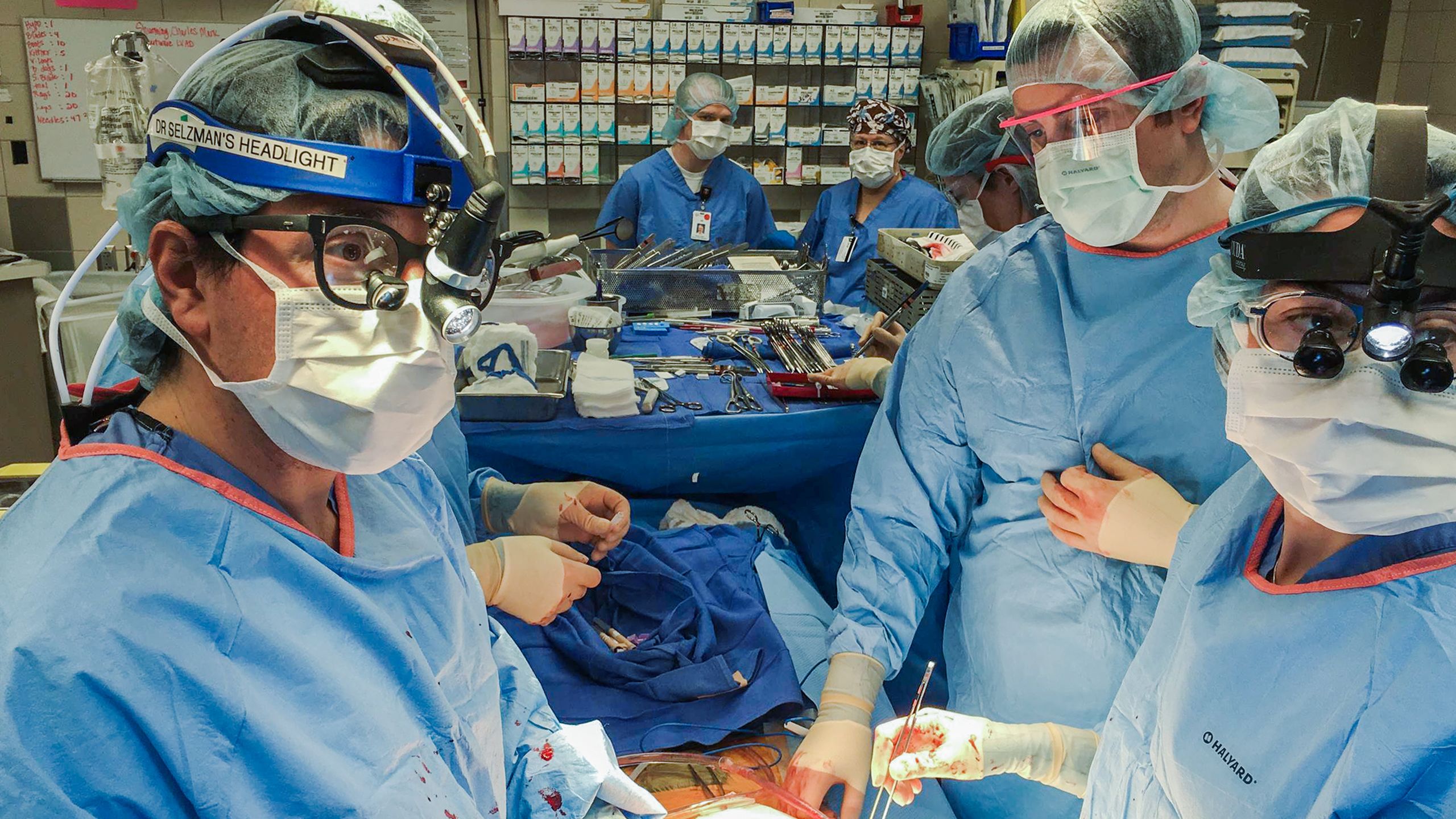
Selzman set about ramping up the LVAD program by building a multidisciplinary team and engaging in innovative clinical trials to test newer, lighter, implantable LVADs.
The number of LVAD implants at University Hospital exponentially climbed over the ensuing years, placing U of U Health as a leading institution in the field.
Over the years, newer LVAD technologies have transformed how physically intrusive they were on patients. Where the earlier iteration was so heavy and bulky that a smaller-figured patient would struggle to walk with it inside them, the current model is so small patients barely notice it, except for a wire protruding from their stomach.
Accordingly, the hospital’s reputation in the heart failure field has continued to grow, and now the institution treats patients who live in Utah and across the country. Drakos, Selzman, and their teams also train other teams in LVADs on regional, national and international levels.
To date, U of U Health cardiothoracic surgeons have implanted more than 1,000 LVADs.
“This is a therapy that’s going, going, going,” Selzman said, a success driven by its remarkable impact on heart disease survival rates.
A patient with end-stage heart failure on medical therapy alone has less than a 10 percent chance of surviving two years. But with an LVAD, that rate jumps as high as 70 percent. “There’s no chemotherapy drug for any cancer out there that takes a survival rate at two years from 10 percent to 70 percent,” Selzman said. “It’s mind-boggling.”

This first-generation LVAD HeartMate XVE was large and heavy. It was positioned in the abdomen next to the stomach.
This first-generation LVAD HeartMate XVE was large and heavy. It was positioned in the abdomen next to the stomach.

The HeartMate II was a second-generation device that was smaller and more comfortable than 1st generation VADs. Rather than working as a pump, the device provided continuous blood flow.
The HeartMate II was a second-generation device that was smaller and more comfortable than 1st generation VADs. Rather than working as a pump, the device provided continuous blood flow.
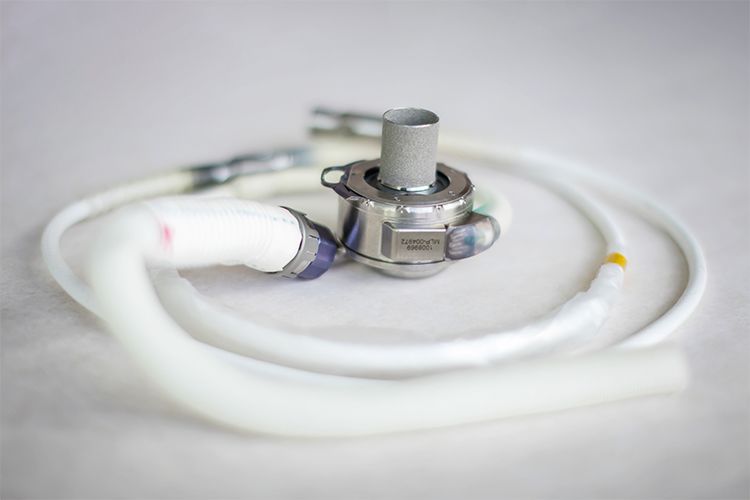
The HeartMate 3 is a third-generation device. It is now the only FDA approved LVAD on the market (at time of publishing).
The HeartMate 3 is a third-generation device. It is now the only FDA approved LVAD on the market (at time of publishing).
Over the years, newer LVAD technologies have transformed how physically intrusive they were on patients. Where the earlier iteration was so heavy and bulky that a smaller-figured patient would struggle to walk with it inside them, the current model is so small patients barely notice it, except for a wire protruding from their stomach.

This first-generation LVAD HeartMate XVE was large and heavy. It was positioned in the abdomen next to the stomach.

The HeartMate II was a second-generation device that was smaller and more comfortable than 1st generation VADs. Rather than working as a pump, the device provided continuous blood flow.
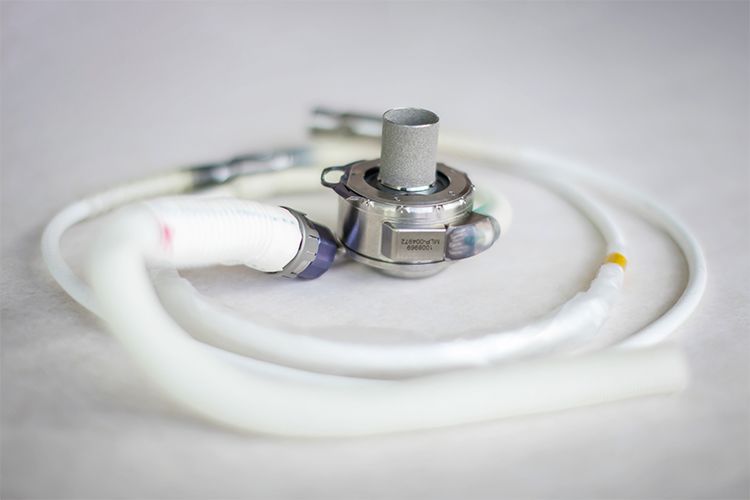
The HeartMate 3 is a third-generation device. It is now the only FDA approved LVAD on the market (at time of publishing).
Accordingly, the hospital’s reputation in the heart failure field has continued to grow, and now the institution treats patients who live in Utah and across the country. Drakos, Selzman, and their teams also train other teams in LVADs on regional, national and international levels.
To date, U of U Health cardiothoracic surgeons have implanted more than 1,000 LVADs.
“This is a therapy that’s going, going, going,” Selzman said, a success driven by its remarkable impact on heart disease survival rates.
A patient with end-stage heart failure on medical therapy alone has less than a 10 percent chance of surviving two years. But with an LVAD, that rate jumps as high as 70 percent. “There’s no chemotherapy drug for any cancer out there that takes a survival rate at two years from 10 percent to 70 percent,” Selzman said. “It’s mind-boggling.”

This first-generation LVAD HeartMate XVE was large and heavy. It was positioned in the abdomen next to the stomach.
This first-generation LVAD HeartMate XVE was large and heavy. It was positioned in the abdomen next to the stomach.

The HeartMate II was a second-generation device that was smaller and more comfortable than 1st generation VADs. Rather than working as a pump, the device provided continuous blood flow.
The HeartMate II was a second-generation device that was smaller and more comfortable than 1st generation VADs. Rather than working as a pump, the device provided continuous blood flow.

The HeartMate 3 is a third-generation device. It is now the only FDA approved LVAD on the market (at time of publishing).
The HeartMate 3 is a third-generation device. It is now the only FDA approved LVAD on the market (at time of publishing).
“Coming Back From the Dead”
The success of U of U Health’s LVAD program got Drakos, Selzman and others thinking, “What can we do to make the biology of the heart muscle work better?” Selzman said. To answer the question, Selzman and Drakos built a partnership that linked University Hospital’s cardiology clinic and cardiothoracic surgery suite to the Drakos lab at CVRTI.
When Selzman implanted an LVAD in a heart failure patient that Drakos and their other cardiology partners had been taking care of for an extended period of time in the clinic, he’d cut out a small piece of heart muscle from the left ventricle in order to make way for the mechanical support device to be placed into the heart. That human heart tissue, which would have otherwise been thrown away, was collected by Drakos and his team from the surgical operating room and brought to the research laboratory. Selzman, Drakos and the LVAD program’s nurse coordinators also closely followed each LVAD-implanted patient to see how they ultimately fared.
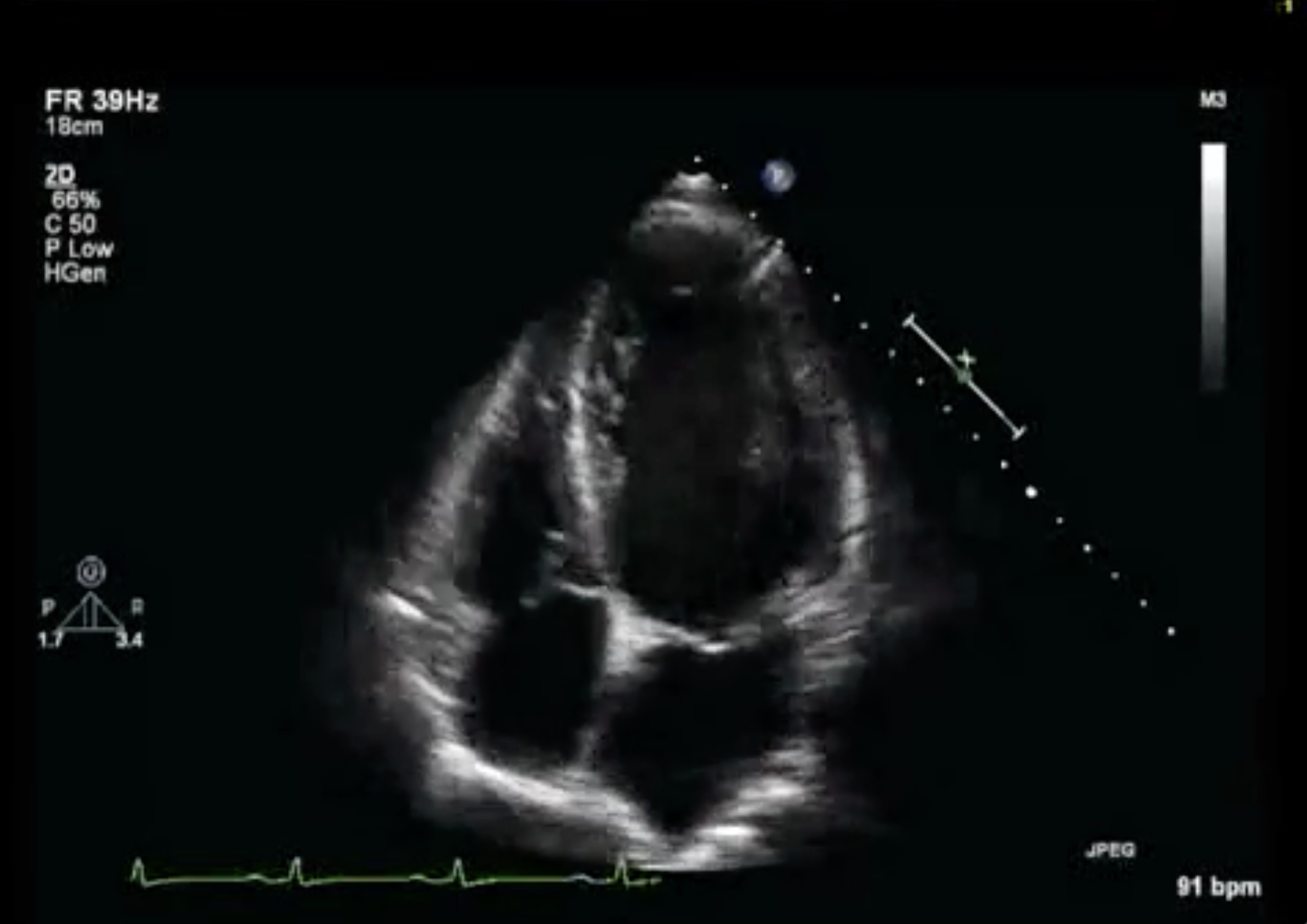
By analyzing the extracted heart tissue in the lab together with the patient’s echocardiogram data, they could benchmark the heart’s physiology at the time the LVAD was implanted and then evaluate how the organ changed after being supported by the device.
What they discovered was that after receiving the LVAD, some patients’ hearts actually got much better. This had become apparent to Selzman in the OR as well. Using the LVAD in its intended role as a temporary stop-gap until a donor heart becomes available for transplant, Selzman would open a patient up and discover that they no longer needed it because their own heart had in the meantime recovered.
“Why are we using this new heart on somebody that actually has a heart that works?” Selzman thought.
That proved to be the clinical inspiration for their research moving forward and has been foundational to one of CVRTI and University of Utah Health’s current claims to fame in cardiovascular disease research, Selzman argued.
In October 2020, Drakos, Selzman, and other CVRTI researchers and collaborators published a report demonstrating what they had seen in the OR: the unexpected role that LVADs could play in supporting the healing of failing hearts.
Among heart failure patients who saw enough improvement that they could have their LVAD removed, almost 80 percent of the patients were still alive five years later, a remarkably higher rate than normal for patients who had end-stage heart failure.
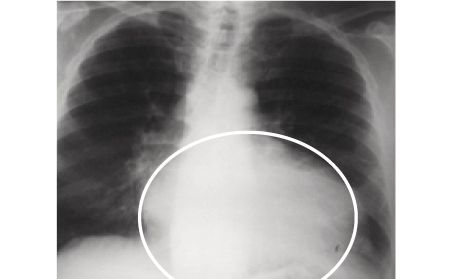
X-ray of a patient with advanced heart failure before an LVAD was implanted. The heart is enlarged significantly.
X-ray of a patient with advanced heart failure before an LVAD was implanted. The heart is enlarged significantly.
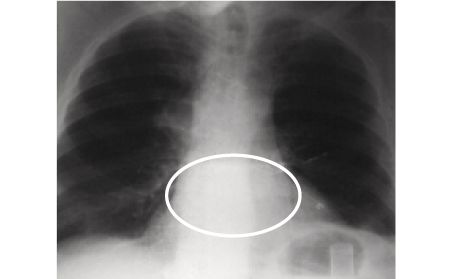
After 14 weeks with an LVAD, the heart shrank to normal size and heart function improved.
After 14 weeks with an LVAD, the heart shrank to normal size and heart function improved.
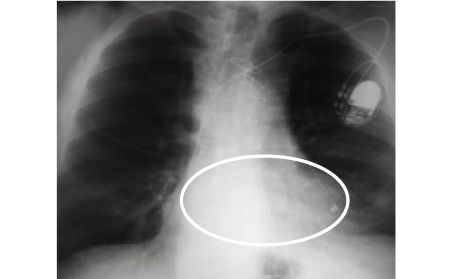
7 years after the LVAD is removed, heart recovery is sustained.
7 years after the LVAD is removed, heart recovery is sustained.
The success of U of U Health’s LVAD program got Drakos, Selzman and others thinking, “What can we do to make the biology of the heart muscle work better?” Selzman said. To answer the question, Selzman and Drakos built a partnership that linked University Hospital’s cardiology clinic and cardiothoracic surgery suite to the Drakos lab at CVRTI.
When Selzman implanted an LVAD in a heart failure patient that Drakos and their other cardiology partners had been taking care of for an extended period of time in the clinic, he’d cut out a small piece of heart muscle from the left ventricle in order to make way for the mechanical support device to be placed into the heart. That human heart tissue, which would have otherwise been thrown away, was collected by Drakos and his team from the surgical operating room and brought to the research laboratory. Selzman, Drakos and the LVAD program’s nurse coordinators also closely followed each LVAD-implanted patient to see how they ultimately fared.
By analyzing the extracted heart tissue in the lab together with the patient’s echocardiogram data, they could benchmark the heart’s physiology at the time the LVAD was implanted and then evaluate how the organ changed after being supported by the device.
What they discovered was that after receiving the LVAD, some patients’ hearts actually got much better. This had become apparent to Selzman in the OR as well. Using the LVAD in its intended role as a temporary stop-gap until a donor heart becomes available for transplant, Selzman would open a patient up and discover that they no longer needed it because their own heart had in the meantime recovered.

X-ray of a patient with advanced heart failure before an LVAD was implanted. The heart is enlarged significantly.

After 14 weeks with an LVAD, the heart shrank to normal size and heart function improved.

7 years after the LVAD is removed, heart recovery is sustained.
“Why are we using this new heart on somebody that actually has a heart that works?” Selzman thought.
That proved to be the clinical inspiration for their research moving forward and has been foundational to one of CVRTI and University of Utah Health’s current claims to fame in cardiovascular disease research, Selzman argued.
In October 2020, Drakos, Selzman, and other CVRTI researchers and collaborators published a report demonstrating what they had seen in the OR: the unexpected role that LVADs could play in supporting the healing of failing hearts.
Among heart failure patients who saw enough improvement that they could have their LVAD removed, almost 80 percent of the patients were still alive five years later, a remarkably higher rate than normal for patients who had end-stage heart failure.

X-ray of a patient with advanced heart failure before an LVAD was implanted. The heart is enlarged significantly.
X-ray of a patient with advanced heart failure before an LVAD was implanted. The heart is enlarged significantly.

After 14 weeks with an LVAD, the heart shrank to normal size and heart function improved.
After 14 weeks with an LVAD, the heart shrank to normal size and heart function improved.

7 years after the LVAD is removed, heart recovery is sustained.
7 years after the LVAD is removed, heart recovery is sustained.


“People initially considered this like coming back from the dead, like Lazarus, like a miracle,” Drakos said. “And miracles, as we know, don’t happen every day.”
Only a subset of heart failure patients improve their hearts after LVAD support, and Drakos’ lab is teaming with biochemists and cell biologists at U of U Health to fully understand the properties and mechanisms that promote heart recovery.
His goal is to use this knowledge to develop new therapeutics that heal the human heart, therefore limiting the need for mechanical circulatory support.
Indeed, in 2021, they published in top-tier scientific journals their research findings identifying novel therapeutic targets such as MCT4 and VDAC2 that are now being pursued as new, groundbreaking therapies for heart failure.
Enough Is Enough
With Shaw’s arrival in 2019 to take the reins of running CVRTI, the emphasis of the institute has shifted toward bench-to-bedside research. This includes Shaw’s own passionate commitment as a physician-scientist to a molecule called cardiac BIN1 and the role it may play in healing a damaged heart muscle through gene therapy.
Shaw’s scientific partner in that work is TingTing Hong, MD, PhD, an independent investigator at CVRTI who grew up on the southeast coast of China, three hours south of Shanghai. Members of her family had histories of stroke and coronary artery disease, but it was her adored 72-year-old grandfather’s death that particularly troubled her. He had been diagnosed with heart failure but refused to attend the nearest hospital. Instead, each time he had an episode, he went to a local rural clinic with few resources. Impatiently, he accelerated the IV drip speed of his medication, which led to his demise.


The devastating experience led Hong to train to become a physician at Peking University. From there, she attended University of Michigan for a PhD in cardiovascular pharmacology before digging into molecular mechanisms and cell biology.
That led her to Shaw’s lab in 2007, when he was at University of California, San Francisco, to study the cell biology of calcium channel trafficking. She focused on L-type calcium channels, which are key to normal heart contraction, a role dramatically reduced by heart failure.
Hong started her own research lab in 2013, continuing her long-time collaboration with Shaw and his team to answer the question: Could they restore the function of heart muscle cells during heart failure by repairing the architecture of those cells?


Shaw and Hong burrowed down into a key heart cell protein they discovered and named cardiac BIN1, the levels of which decreased in a failing heart. What they found was if the protein was directly injected into failing hearts in mice, their condition could be reversed.
When Shaw was asked to take over CVRTI, he jumped at the chance, in part because of its storied legacy but also because its well-established research program meant he and his team could “actually move this therapy forward.”
What drives Shaw is the tragic reality he often witnessed daily in the ICU. Patients who, because they couldn’t get a heart transplant—whether for lack of availability or medical complications—were essentially stranded in their beds, such as one man who is never far from his mind.
The patient was in heart failure in one of the top CVICUs in the country, where he had spent several months tethered to machinery keeping him alive. He knew he was a burden on his family and that he would not improve. Medical staff had put in a new type of temporary LVAD and waited for the next step.
But if staff hadn’t given up, the patient had. He did not want to burden his family further, economically or emotionally. He looked into Shaw’s eyes. “They need to move on with their lives,” he said. “I’ve had enough. Thank you. Stop the therapies.”
His family disagreed with him but respected his decision. Therapy was discontinued, and he died the next morning. “Damn it, we suck!” Shaw thought afterward. Shaw had nothing in his medical arsenal he could offer to either improve his heart or dissuade him from his decision. “We have to nod our heads in sage agreement that it’s enough and that’s a rational decision,” he said.
Next Generation
In summer 2022, Shaw and Hong were in the midst of the next step after their successes with mice to move their gene therapy forward, running a test with three pigs. Two had been treated with cBIN1 gene therapy; the third, a negative control that had simply been given saline. Shaw was traveling to visit family when Hong called to consult on the control animal whose heart was failing.
“How are the other pigs doing?” he asked.
She had recently checked their echocardiograms. Their hearts had normalized in size, as had the rate at which blood pumped from their hearts. “Oh, they’re normal,” she said.
Shaw was silent for a moment. They had been expecting some reversal of the condition but certainly not normalizing of the heart. He was almost as shocked by how casual Hong had been in delivering the news as he was by the news itself.
“Do you understand what you’re saying?” Shaw said. “Do you realize how important this data is?”
“Of course,” Hong said. “It works.” She had always had faith the project would work.
After they hung up, Shaw sat in silence for an hour in the lengthening shadows of the bedroom, sifting through the enormity of what he had just learned after 15 years of working toward this one, priceless moment.

Shaw’s reaction to her news made Hong think about her grandfather—and about all the patients in ICUs in China she’d treated who had died of heart failure for lack of a transplant.
Perhaps they could one day treat heart failure in the ICU—and perhaps treat early-stage heart failure so patients wouldn’t have to even enter the ICU. The gene therapy that Hong and Shaw are developing would be far less invasive—and could be much more effective—than machines like the artificial heart and LVAD.
“It sounds remarkable, but that’s what the animals are telling us,” Shaw said. “People will walk out of the ICU.” As much as he knew that further testing needed to be done—and how careful he had to be about wildly over-emphasizing their initial findings—he felt that same sense of faith that Hong felt. Perhaps he’d always had it—but hadn’t articulated it to himself of late.
Forty years on from Barney Clark’s life-changing operation, the legacy that was the gift of his own life for the furtherance of medical science remained as vibrant as ever. It was a gift that, four decades ago, former University of Utah President Chase Peterson told reporters stemmed from Clark’s faith that something good would come out of it for patients with failing hearts. “If other people do get a quality of life that is more obviously satisfactory,” Peterson said, “they better thank Barney Clark.”
That same faith continues today, whether in the confident hands and hearts of Selzman, Drakos, Shaw, and Hong or all the other heart researchers, physicians, and support staff at U of U Health over the decades.
A faith that their interdisciplinary efforts in cardiovascular research—to gain a deeper understanding of the heart and use that knowledge to create new tests and treatments—will indeed change people’s lives for the better.
Heart Research and Innovation at U of U Health
The innovation that led to Barney Clark’s artificial heart transplant is one thread of so many more in the history of U of U Health’s and its partners’ commitment to remaining on the frontline of cardiovascular care. Below is a timeline of U of U Health’s discoveries that have had a powerful impact on understanding and advancing treatment of cardiovascular disorders, and improving the health of cardiac patients worldwide.
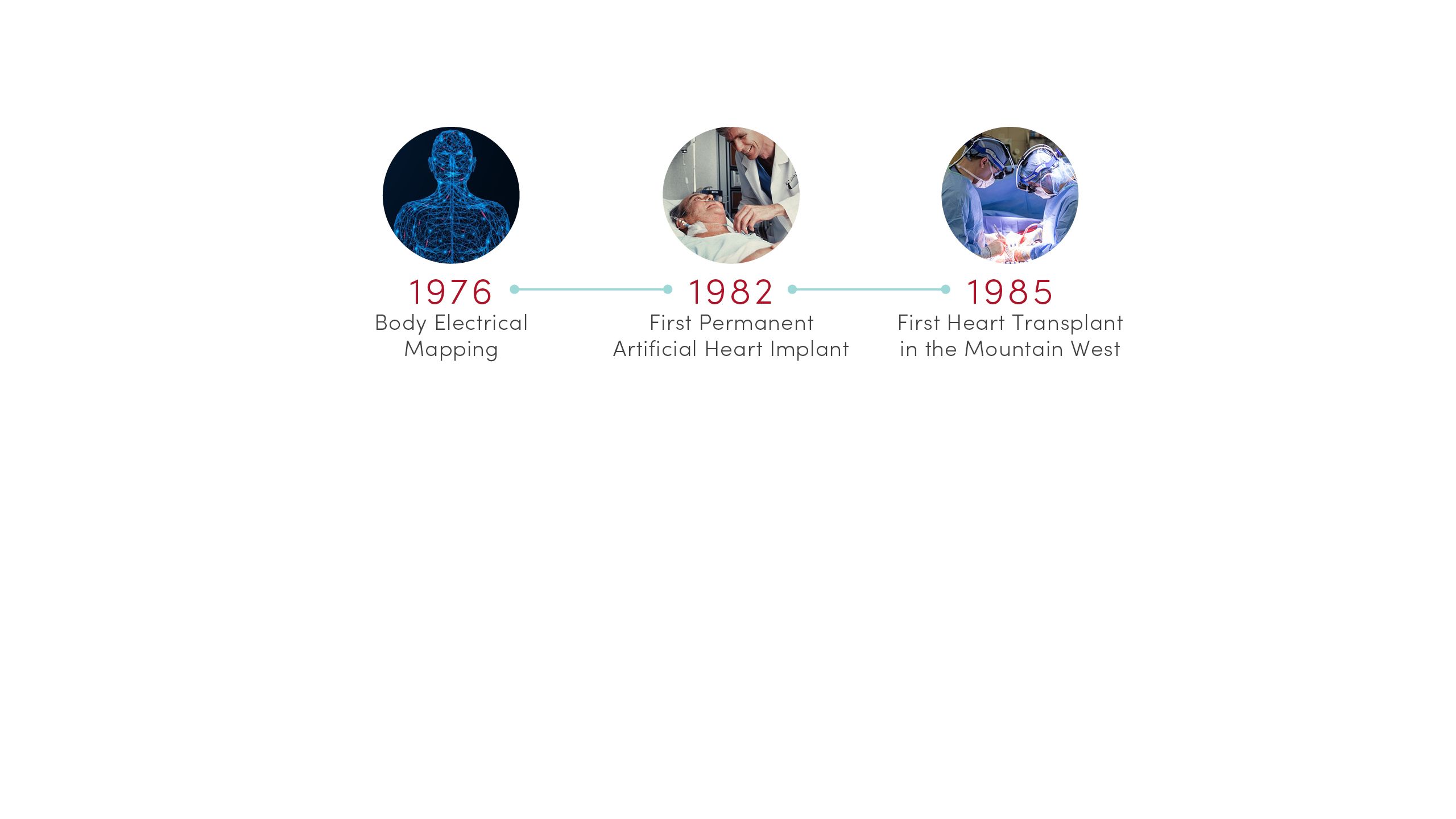
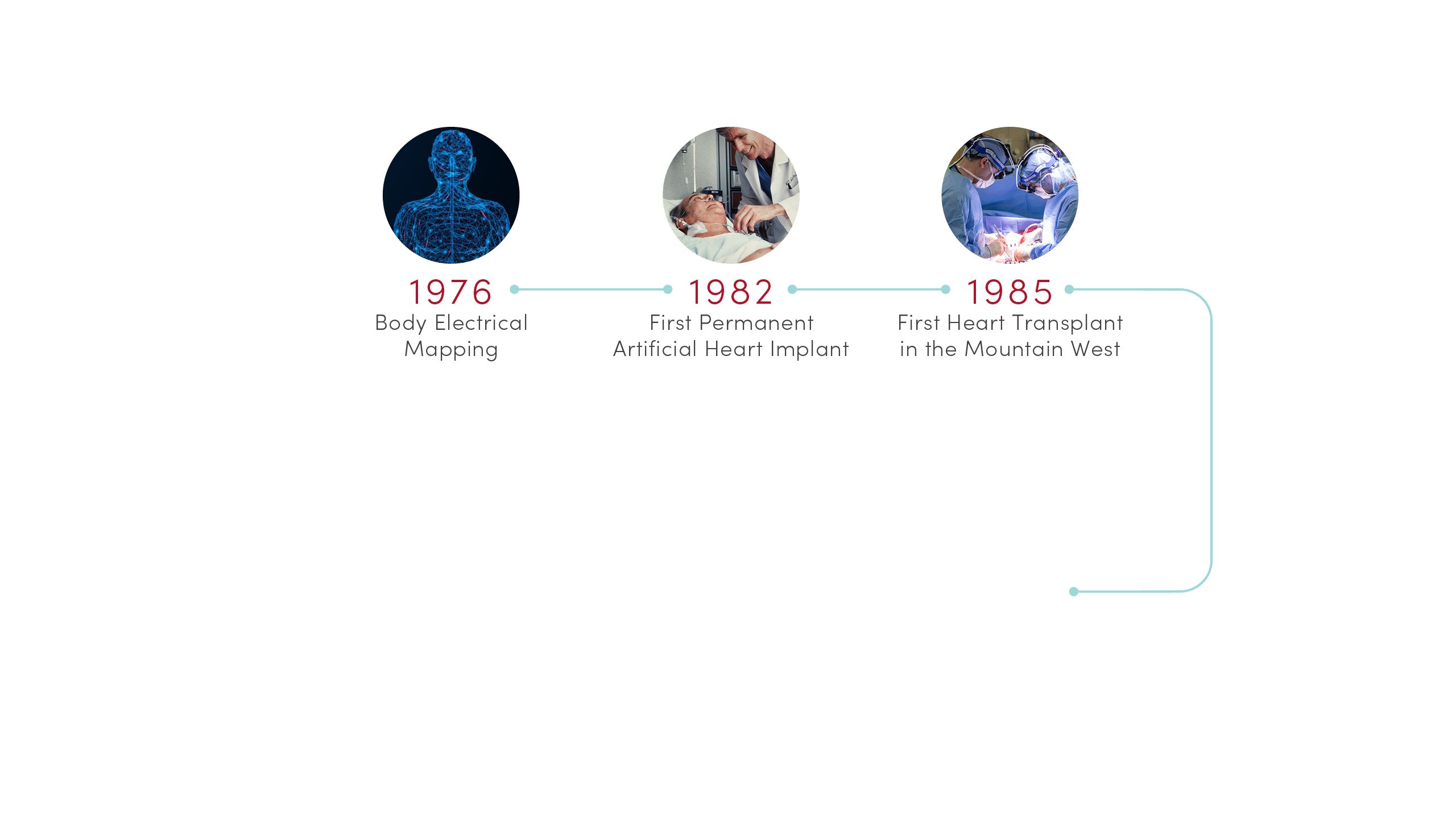
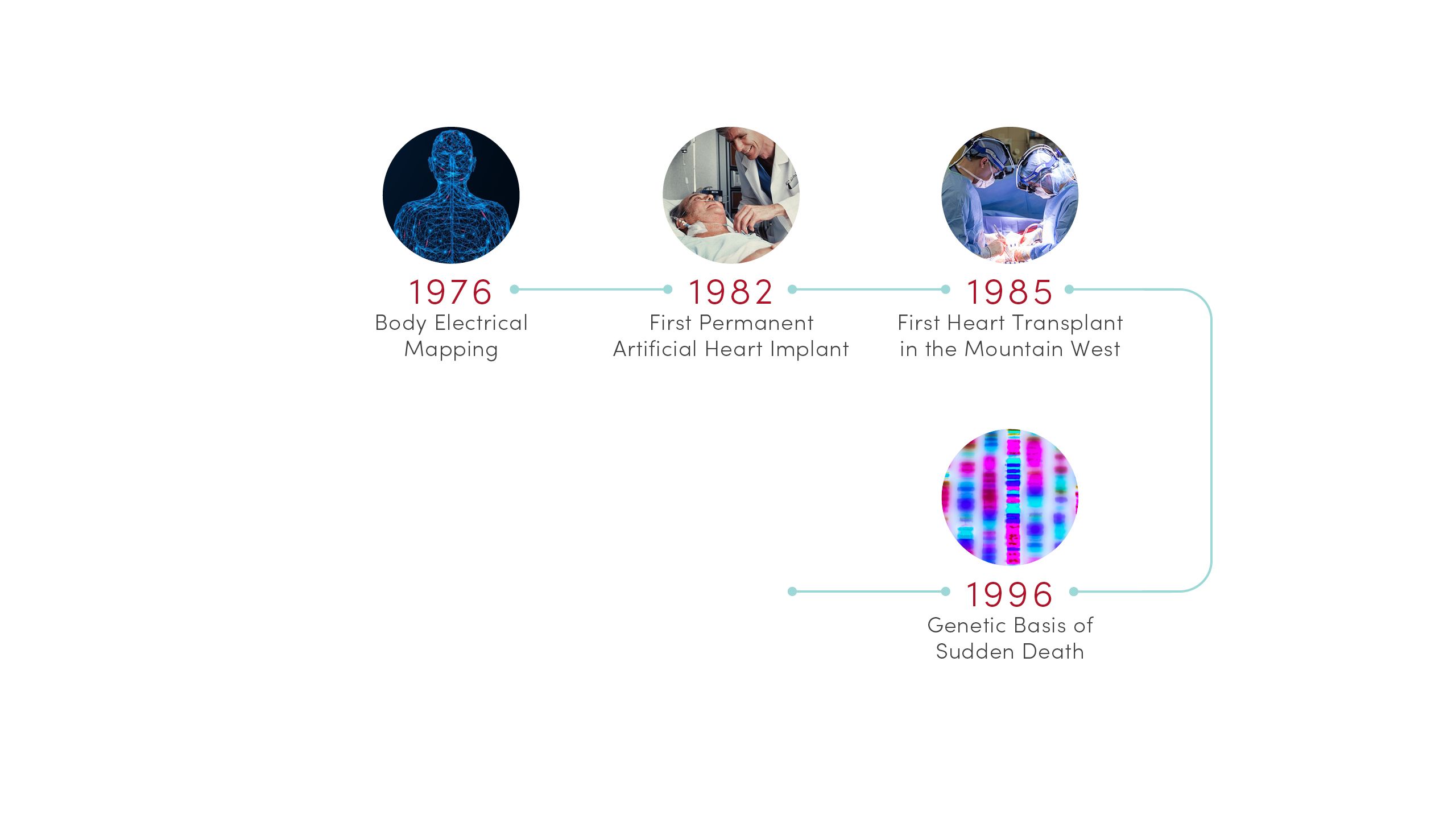
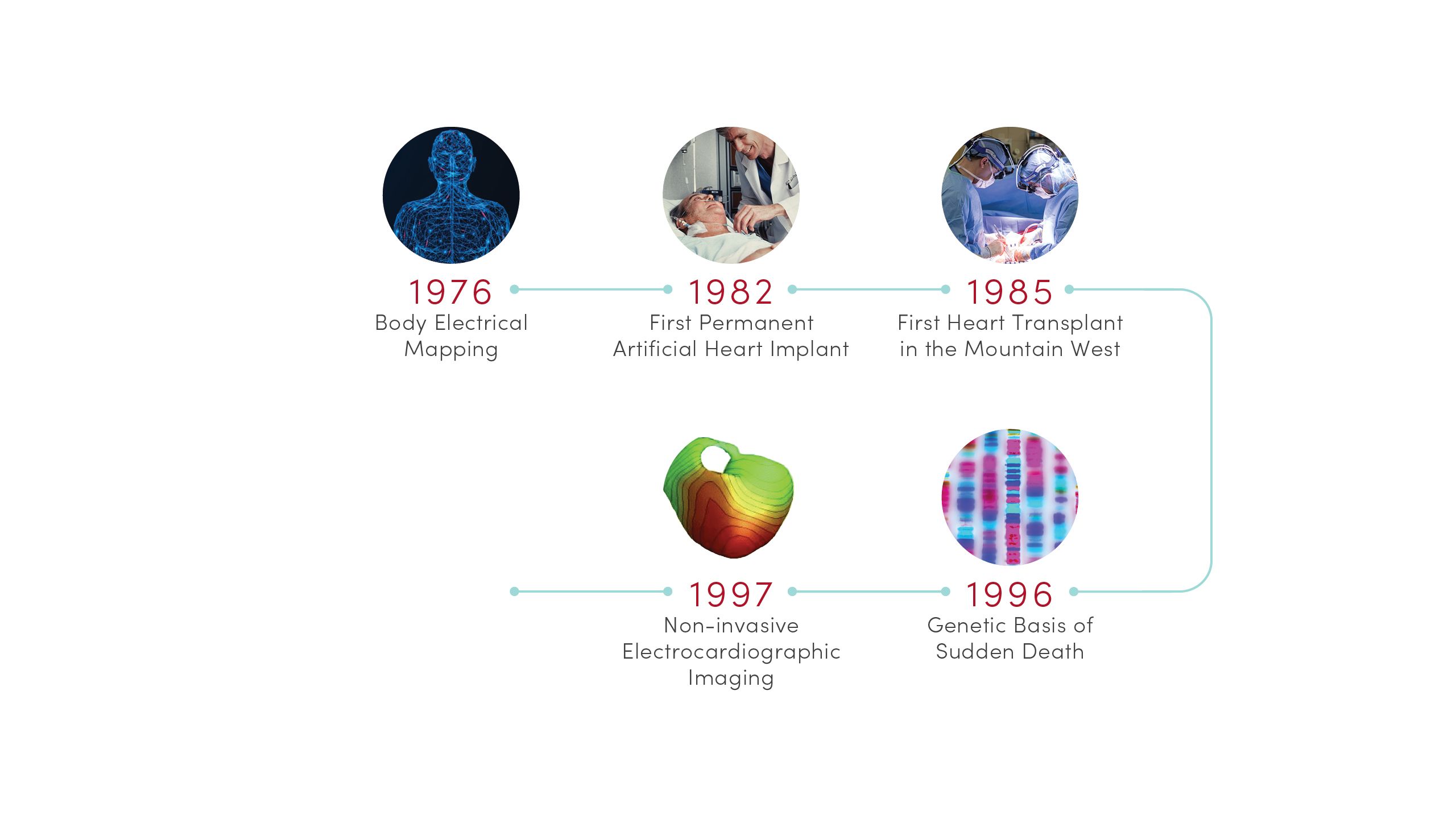
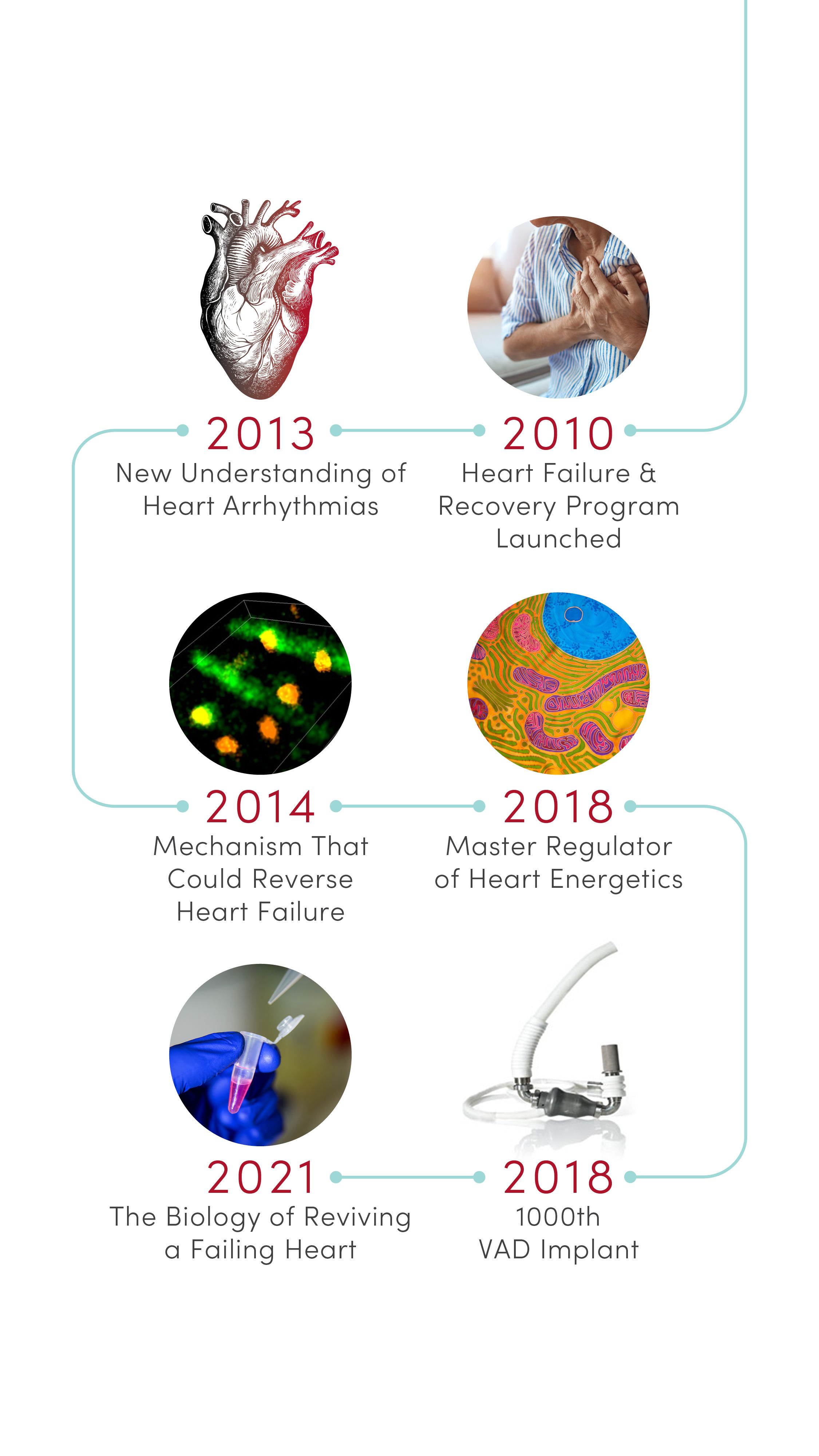
1976 | Body Electrical Mapping
CVRTI scientists performed the first in-human electrical mapping, leading to a new understanding of how the heart transmits electrical signals. The advance laid the theoretical groundwork for sophisticated cardiac electrical mapping and ablation that is now used to treat patients with abnormal heart rhythms.
1982 | First Permanent Artificial Heart Implant
University of Utah Hospital surgeons implanted the first total artificial heart for long-term use in patient Barney Clark. A team of scientists led by Willem Kolff, MD, PhD, at the U’s Division of Artificial Organs and Biomedical Engineering developed the concept of the Jarvik-7 heart.
1985 | First Heart Transplant in the Mountain West
University of Utah Hospital surgeons provided the region’s first living heart transplant to 16-year-old Tony Shepherd. This led to the nationally acclaimed Utah Transplant Affiliated Hospitals (U.T.A.H.) Cardiac Transplant Program that has transplanted hearts in more than 1,650 patients to date.
1996 | Genetic Basis of Sudden Death
Researchers at U of U Health’s Eccles Institute of Human Genetics discovered causes of long QT syndrome, a condition that can lead to an abnormal heartbeat and sudden death. These advances laid the groundwork for preventives and treatments that are saving lives today.
1997 | Non-invasive Electrocardiographic Imaging
Demonstrated that recordings from the body’s surface can reliably detect 3D electrical activation of the heart’s ventricles. Based in part on this discovery, physicians can now locate and eliminate sources of heart arrhythmia with targeted radiation.
2002 | Basis of Arrhythmia in Andersen-Tawil Syndrome
Identified novel genetic mutations in potassium channels that are essential for transmitting electrical signals required for the heart to beat. The discovery provided new insight into the mechanisms of cardiac arrhythmia in patients with Andersen-Tawil syndrome.


2010 | Heart Failure & Recovery Program Launched
Built on advances in research, U of U Health launched the nationally recognized Heart Failure & Recovery Program. Patients take part in an innovative therapy that promotes heart recovery in heart failure patients, where the heart rests with transient use of a left ventricular assist device (LVAD) or other mechanical circulatory support (MCS) device.
2013 | New Understanding of Heart Arrhythmias
Identified a protein, GJA1-20k, required by heart cells to transmit the electrical signals needed for a regular heartbeat. Based on this discovery, CVRTI scientists are now developing a new therapy for patients with certain severe arrhythmia syndromes.
2014 | Mechanism That Could Reverse Heart Failure
Determined that raising levels of a protein called cBIN1 can strengthen the heart and reverse heart failure in animal models. Based on this groundbreaking research, CVRTI scientists are developing a novel gene therapy for testing in clinical trials.
2018 | Master Regulator of Heart Energetics
Identified a protein, SMYD1, that is a potent regulator of heart metabolism and cardiac energetics. The discovery could lead to novel treatments to strengthen heart function.
2018 | 1000th VAD Implant
U of U Health cardiothoracic surgeons performed their 1000th ventricular assist device (VAD) implantation, a milestone that marks the significant progress and success of mechanical devices in helping heart failure patients to lead better lives.
2021 | The Biology of Reviving a Failing Heart
Identified characteristics that give some patients the surprising ability to rebound from advanced heart failure. This advance is allowing CVRTI scientists to identify molecular pathways that can inform new types of treatments.
Heart Research and Innovation at U of U Health
The innovation that led to Barney Clark’s artificial heart transplant is one thread of so many more in the history of U of U Health’s and its partners’ commitment to remaining on the frontline of cardiovascular care. Below is a timeline of U of U Health’s discoveries that have had a powerful impact on understanding and advancing treatment of cardiovascular disorders, and improving the health of cardiac patients worldwide.
1976 | Body Electrical Mapping
CVRTI scientists performed the first in-human electrical mapping, leading to a new understanding of how the heart transmits electrical signals. The advance laid the theoretical groundwork for sophisticated cardiac electrical mapping and ablation that is now used to treat patients with abnormal heart rhythms.
1982 | First Permanent Artificial Heart Implant
University of Utah Hospital surgeons implanted the first total artificial heart for long-term use in patient Barney Clark. A team of scientists led by Willem Kolff, MD, PhD, at the U’s Division of Artificial Organs and Biomedical Engineering developed the concept of the Jarvik-7 heart.
1985 | First Heart Transplant in the Mountain West
University of Utah Hospital surgeons provided the region’s first living heart transplant to 16-year-old Tony Shepherd. This led to the nationally acclaimed Utah Transplant Affiliated Hospitals (U.T.A.H.) Cardiac Transplant Program that has transplanted hearts in more than 1,650 patients to date.
1996 | Genetic Basis of Sudden Death
Researchers at U of U Health’s Eccles Institute of Human Genetics discovered causes of long QT syndrome, a condition that can lead to an abnormal heartbeat and sudden death. These advances laid the groundwork for preventives and treatments that are saving lives today.
1997 | Non-invasive Electrocardiographic Imaging
Demonstrated that recordings from the body’s surface can reliably detect 3D electrical activation of the heart’s ventricles. Based in part on this discovery, physicians can now locate and eliminate sources of heart arrhythmia with targeted radiation.
2002 | Basis of Arrhythmia in Andersen-Tawil Syndrome
Identified novel genetic mutations in potassium channels that are essential for transmitting electrical signals required for the heart to beat. The discovery provided new insight into the mechanisms of cardiac arrhythmia in patients with Andersen-Tawil syndrome.

2010 | Heart Failure & Recovery Program Launched
Built on advances in research, U of U Health launched the nationally recognized Heart Failure & Recovery Program. Patients take part in an innovative therapy that promotes heart recovery in heart failure patients, where the heart rests with transient use of a left ventricular assist device (LVAD) or other mechanical circulatory support (MCS) device.
2013 | New Understanding of Heart Arrhythmias
Identified a protein, GJA1-20k, required by heart cells to transmit the electrical signals needed for a regular heartbeat. Based on this discovery, CVRTI scientists are now developing a new therapy for patients with certain severe arrhythmia syndromes.
2014 | Mechanism That Could Reverse Heart Failure
Determined that raising levels of a protein called cBIN1 can strengthen the heart and reverse heart failure in animal models. Based on this groundbreaking research, CVRTI scientists are developing a novel gene therapy for testing in clinical trials.
2018 | Master Regulator of Heart Energetics
Identified a protein, SMYD1, that is a potent regulator of heart metabolism and cardiac energetics. The discovery could lead to novel treatments to strengthen heart function.
2018 | 1000th VAD Implant
U of U Health cardiothoracic surgeons performed their 1000th ventricular assist device (VAD) implantation, a milestone that marks the significant progress and success of mechanical devices in helping heart failure patients to lead better lives.
2021 | The Biology of Reviving a Failing Heart
Identified characteristics that give some patients the surprising ability to rebound from advanced heart failure. This advance is allowing CVRTI scientists to identify molecular pathways that can inform new types of treatments.
Story by Stephen Dark
Design by Jessica Cagle
Photo Credits:
Clinical and U of U Health portraits of Robin Shaw, MD, PhD, Craig Selzman, MD, Stavros Drakos, MD, PhD, and TingTing Hong, MD, PhD, by Charlie Ehlert
Historical photos courtesy of University of Utah Health and Spencer S. Eccles Health Sciences Library
Personal photographs provided by Stavros Drakos, MD, PhD, and TingTing Hong, MD, PhD. Echocardiogram provided by Dipayan Chaudhuri MD, PhD.
Contributors:
Julie Kiefer, PhD—Story editor, timeline author and research advisor
Jesse Colby—Art director
Nick McGregor—Copy editor
Suzanne Winchester—Consultant
